
Discover how AI-engineered nasal antiviral platforms revolutionize flu prevention, enhancing mucosal immunity and offering new treatment possibilities.

Discover how AI-engineered nasal antiviral platforms revolutionize flu prevention, enhancing mucosal immunity and offering new treatment possibilities.

Sarah J. Billups, PharmD, highlights the disconnect between medication adherence metrics and actual patient outcomes, urging a reevaluation of adherence measures in health care.

Orforglipron shows promise in maintaining weight loss for individuals transitioning from injectable therapies, offering a convenient oral option for obesity management.
In patients with ST-segment elevation myocardial infarction, lowering low-density lipoprotein cholesterol to optimal levels prevented neoatherosclerosis, a complication following stent implantation.

The FDA approved the first oral GLP-1 pill for weight management, offering a convenient alternative for obesity treatment and enhancing patient access.
A high-fiber, plant-based diet shows promise in delaying multiple myeloma progression and enhancing metabolism and immune response in patients with precursor conditions.

Prateek Bhatia discusses optimizing staffing and workflows in infusion centers to enhance patient care and reduce delays in treatment.

Tracking your net worth monthly can shift your financial mindset and build lasting wealth.

The FDA approves Accrufer for children 10 and older, offering a well-tolerated solution for iron deficiency, enhancing pediatric health outcomes.
Researchers uncover how type 2 innate lymphoid (ILC2) and T helper 2 (Th2) cells in allergic asthma survive toxic environments, revealing potential new therapeutic strategies for treatment.

IV compounding robotics and next-generation dispensing technology enhance pharmacy workflows, boost staff engagement, and improve patient care outcomes.

A series of workplace factors were found to increase the risk of long COVID, including close contact with colleagues and commuting through public transportation.

Vitamin D is vital for bone health and immune function, yet many Americans remain deficient.

Pharmacy technicians enhance patient safety and efficiency by mastering communication, situational awareness, and leadership skills.

The novel agent has demonstrated efficacy in symptomatic and less severe patients with obstructive hypertrophic cardiomyopathy in thorough phase 3 clinical trials.

Although both sex groups showed cognitive decline, women with chronic kidney disease (CKD) had significantly better cognitive scores than men.
Fam-trastuzumab deruxtecan-nxki (T-DXd) promises enhanced outcomes for HER2-positive early breast cancer patients post-neoadjuvant treatment.

Pharmacists explore new breast cancer therapies and management strategies, enhancing patient care and treatment outcomes at SABCS.
Ribociclib shows higher early treatment modifications than palbociclib in HR+/HER2– breast cancer, emphasizing the need for proactive toxicity management.
Heidi De Souza, MPH, reveals low RSV vaccine uptake among older adults, highlighting disparities and barriers that hinder access and awareness post-FDA approval.
The approval offers hope for improved lung function and new treatment options for patients.
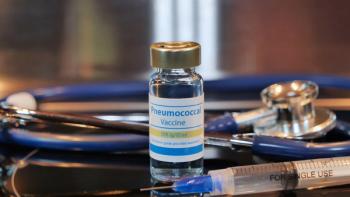
Serotypes included in the pneumococcal 21-valent conjugate vaccine (PCV21) cause outsized disease and economic burden in Norwegian patients with pneumococcal disease.

Lessons learned from interprofessional spiritual training and the evolving role of palliative care pharmacists toward an integrated and patient-centric health care system.

Oncology pharmacists and the pharmaceutical industry can enhance patient care through improved support, education, and access to innovative treatments.
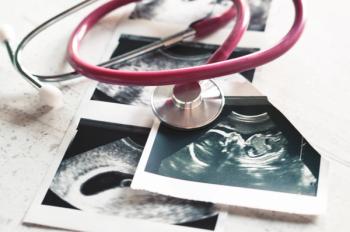
COVID-19 vaccination significantly lowers severe illness and preterm birth risks for pregnant individuals.

The most impactful FDA approvals of 2025 feature groundbreaking treatments for mental health, STIs, diabetes, pain relief, and cardiovascular health.

A technician-driven workflow implemented in a health system’s specialty pharmacy reduced high-cost oncology drug waste.