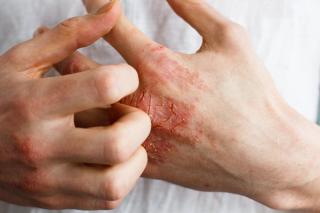
Atopic Dermatitis
Latest News
Latest Videos

Podcasts
CME Content
More News
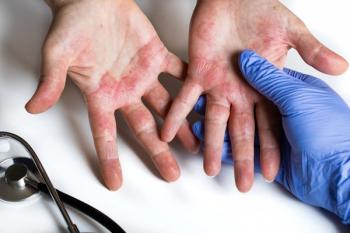
Investigators from the phase 3 COAST 1 trial demonstrated the efficacy and safety of amlitelimab in patients with moderate-to-severe atopic dermatitis.
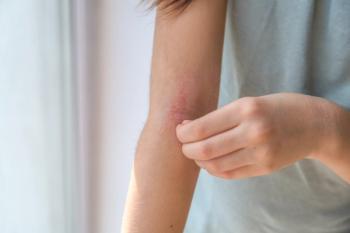
Nemolizumab shows promising long-term efficacy in reducing itch and skin lesions for atopic dermatitis, enhancing quality of life for patients.

Matthew Zirwas, MD, addresses the significant financial burden associated with the medication, emphasizing the importance of pharmacists in navigating prior authorizations and access patient support programs.
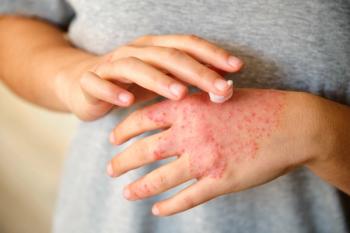
Efficacy and tolerability of topical treatments can vary by region, particularly sensitive areas such as the face and neck.

At week 8, significantly more children, adolescents, and adults who applied 1.5% ruxolitinib cream versus vehicle achieved IGA treatment success.

Embedding a pharmacist in the dermatology clinic improved clinical and financial outcomes.
Rezpegaldesleukin is designed to balance the body’s immune system response through activation of regulatory T-cells.

Navigating Pediatric Atopic Dermatitis: Insights Into Treatment, Challenges, and Care Considerations
Atopic dermatitis significantly impacts quality of life, and requires personalized management through trigger identification, skin barrier maintenance, and a range of topical, oral, and biologic therapies to reduce symptoms and prevent flare-ups.
The monoclonal antibody demonstrates the ability to reduce the incidence and improve symptoms related to sleep disorders and psychiatric disorders such as anxiety.
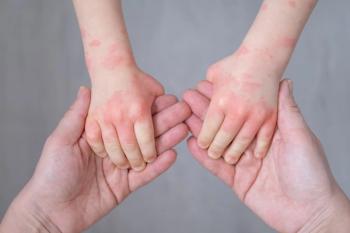
Proven effective in the treatment of atopic dermatitis (AD) and other allergic conditions, these study results confirm the ability of dupilumab to improve symptoms in children with other concurrent conditions.

Nemolizumab exclusively targets IL-31 receptor alpha, inhibiting the signaling of IL-31, which drives disease mechanism in atopic dermatitis.

The approval builds on previous indications for tapinarof, an aryl hydrocarbon receptor agonist, for the topical treatment of atopic dermatitis.

At weeks 16 and 24, approximately 57% and 60% of patients with moderate to severe atopic dermatitis, respectively, achieved at least 75% improvement in Eczema Area and Severity Index score.

If approved, delgocitinib cream would be the first US treatment indicated for moderate to severe chronic hand eczema.

Lebrikizumab (Ebglyss; Eli and Lilly Company) is a monthly maintenance injection with proven efficacy in adults and children aged 12 to 18 years.
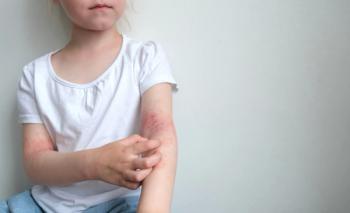
In July 2024, the FDA approved the supplemental new drug application for roflumilast cream 0.15% for patients 6 years and older.
Nemolizumab is the first approved monoclonal antibody specifically inhibiting the signaling of IL-31, which drives disease mechanisms in prurigo nodularis.
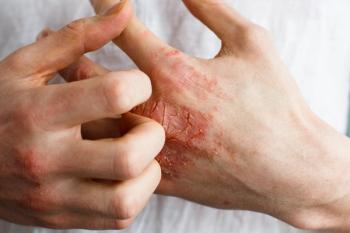
The Lancet published data from DELTA 1 and DELTA 2 trials that displayed treatment success among individuals treated with delgocitinib cream.

Roflumilast is approved as a once daily and steroid-free cream for rapid disease clearance and significant reduction in itch and for long-term disease control.

When initiated at a 15 mg dose and escalated to 30 mg, upadacitinib showed superiority compared with dupilumab, achieving Eczema Area and Severity Score (EASI) 90 at week 16.

Secondhand exposure to e-cigarette smoke may increase risk of pediatric atopic dermatitis in children.

The autoinjector will provide another option for adults in addition to the prefilled syringe that is currently available.

Parents and caregivers sometimes have concerns about their children receiving these injections, but researchers and experts assure that the FDA-approved biologic is safe and effective in young populations.
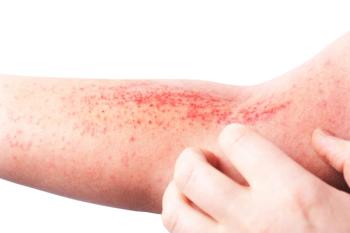
Corticophobia is defined as a phobia of topical corticosteroids due to negative feelings or beliefs, which can come from misinformation about the long-term effects.

The acceptance is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials, as well as interim results from the phase 3 ADORING 3 open-label, long-term extension trial.