
Pharmacists play a vital role in countering GLP-1 misinformation.

Kennedy Ferruggia is an associate editor at Pharmacy Times®. She graduated from The College of New Jersey in 2023 in journalism and marketing. Prior to this position, she worked as a pharmacy associate for community pharmacies.

Pharmacists play a vital role in countering GLP-1 misinformation.

India has confirmed 2 Nipah infections in West Bengal as surveillance tracks 196 contacts with no spread, with the World Health Organization outlining symptoms, transmission, and response measures.

Changes to the federal childhood vaccine schedule have created confusion and variation in practice, placing added responsibility on clinicians and pharmacists to educate patients and ensure evidence-based care.

Phase 1 data show oral NSD2 inhibitor KTX-1001 delivers durable disease control in high-risk myeloma, with manageable thrombocytopenia and combo plans.

COVID-19 significantly impacts diagnosis rates for depression, asthma, and osteoporosis, revealing health care inequities and the need for improved monitoring.

KTX-1001, a novel oral inhibitor of NSD2, demonstrates early clinical activity and manageable safety in heavily pretreated multiple myeloma.

Pharmacists play a critical role in improving access to and affordability of GLP-1 therapies.

Research reveals that patients with asthma who experience symptoms of depression show elevated BDNF levels, suggesting unique biological links between asthma severity and depressive symptoms.

RegeneCyte's HPC therapy shows promise in treating long COVID symptoms, with 85% of patients reporting fatigue relief.

The FDA approved the first dual-agent eye drops for presbyopia, offering a groundbreaking solution for age-related near vision loss.

David Shusterman, MD, reveals how reducing alcohol enhances GLP-1 therapy, improving insulin regulation, kidney health, and overall metabolic outcomes.

GLP-1 receptor agonist effectiveness and tolerability can be significantly improved through targeted pharmacist counseling on alcohol reduction.

New research reveals how specific gut bacteria in infants may reduce the risk of allergies and asthma, offering potential preventive strategies.

Reducing alcohol intake alongside GLP-1 therapy may significantly improve outcomes for patients with obesity or diabetes risk.

Research reveals COVID-19's lasting effects on brain health, highlighting cognitive impairments in patients with long COVID even after recovery.
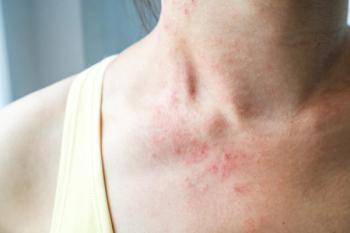
Rocatinlimab shows promise in treating moderate-to-severe atopic dermatitis, offering significant symptom relief.

Insufficient sleep significantly impacts life expectancy, highlighting the need to prioritize quality rest for better health and longevity.
The FDA approves gepotidacin as a groundbreaking oral treatment for uncomplicated urogenital gonorrhea.

Heidi De Souza discusses strategies to enhance RSV vaccine uptake among older adults, emphasizing awareness, coadministration patterns, and public health efforts.
The glucagon-like peptide-1 (GLP-1) receptor agonist medications evaluated included semaglutide, tirzepatide, and liraglutide.
Teclistamab-cqyv shows promising results in treating refractory multiple myeloma, significantly improving survival rates and offering new hope for patients.
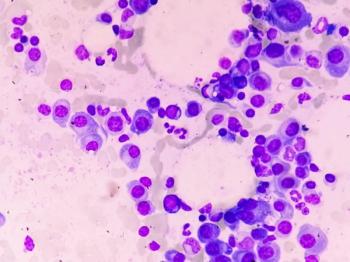
New research uncovers why multiple myeloma affects men more than women, highlighting sex-based differences in disease presentation and risk factors.

Research reveals how eczema's timing and severity influence children's chances of outgrowing food allergies, highlighting the connection between skin health and allergy tolerance.

Long-term type 2 diabetes significantly increases cardiovascular disease risk, revealing potential biomarkers for early intervention and prevention strategies.
A new dual-antibody therapy shows promise in treating extramedullary multiple myeloma, offering hope for patients with limited options.
Bepirovirsen shows promise as a groundbreaking treatment for chronic hepatitis B, achieving significant functional cure rates in recent phase 3 trials.

Pharmacists enhance community health through outreach programs, offering vaccinations, health screenings, and education to improve access and trust in health care.

Gestational diabetes disrupts RNA splicing in the placenta, revealing potential targets for improving pregnancy outcomes.

Psychiatric pharmacists enhance substance use disorder (SUD) care through interdisciplinary collaboration, advocating for policy changes to improve patient outcomes and access to treatment.

Psychiatric pharmacists enhance opioid use disorder treatment through innovative protocols, collaboration, and education.

Published: June 12th 2025 | Updated: June 16th 2025
Published: June 3rd 2025 | Updated: June 11th 2025
Published: March 10th 2025 | Updated: March 21st 2025

Published: March 7th 2025 | Updated: March 10th 2025

Published: December 18th 2024 | Updated: January 2nd 2025

Published: July 26th 2024 | Updated: