The new sFIS assay may be capable of identifying immune biomarkers indicative of survival chances and treatment effectiveness for patients with ovarian cancer.

The new sFIS assay may be capable of identifying immune biomarkers indicative of survival chances and treatment effectiveness for patients with ovarian cancer.
Treatment for endometrial cancer includes surgery, radiation therapy, chemotherapy, immunotherapy, and supportive care.
In the IMPACT trial, women who were positive for high-risk HPV received a follow-up triage test to help determine whether their cervical cells were transforming to cervical pre-cancer.

This weight loss can prevent the onset of type 2 diabetes for new mothers.

Diurex Water Capsules treat bloating, swelling, and other signs of water weight gain related to menstrual symptoms.
Endometriosis most commonly involves the ovaries, fallopian tubes, and the tissue lining the pelvis.

In a report, the World Health Organization and a partner ask developers, funders, and investigators to accelerate developing a vaccination.
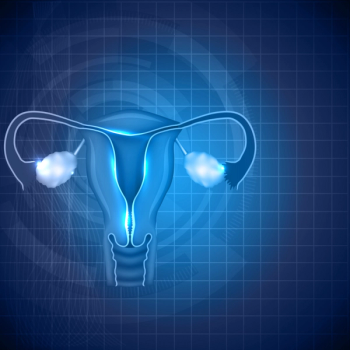
An ectopic pregnancy occurs when a fertilized egg implants and grows outside the main cavity of the uterus.

Women leadership roles and mentorship is extremely important in the pharmacy field, and this week's episode features Dr. Andrea Collaro, Walgreens Boots Alliance, Senior Director, Brand Management/Product Development, Owned Brands Health & Wellness, who goes into more specifics.
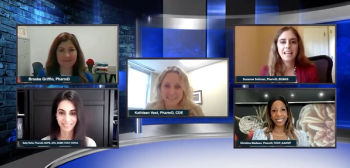
Expert panelists delve into topics related to women's health and women's health care during the COVID-19 pandemic, including pregnancy and COVID-19 vaccine considerations and best practices for guiding decisions on birth control.

Study indicates that women with trauma had greater white matter hyperintensities (WMH) than women without trauma, and the most common trauma associated with WMH was sexual violence.

Nathan Bryson, PhD, chief scientific officer of Field Trip Health, said the company's new psychedelic compound, FT-104, could have potential uses in the treatment of postpartum depression.

Common and debilitating, the mental health condition is often underdiagnosed and undertreated.


Azo treats urinary pain, burning, urgency, and frequency associated with urinary tract infections.

The investigators found that the rate of preterm birth in pregnant women who tested positive for SARS-CoV-2 was a function of the severity of infection.

If the research is confirmed, it could be a warning sign for women to make diet and lifestyle changes aimed at preventing cardiovascular disease.

Ibrexafungerp is a triterpenoid antifungal drug for adult and post-menarchal pediatric females with vulvovaginal candidiasis.

Kelsey Ramsden, CEO of Mind Cure Health, said research into the use of MDMA-assisted therapy could have a huge impact on the treatment of female hypoactive sexual desire disorder.
Adults 40 to 59 years of age should consult their clinician before taking aspirin, and adults 60 years of age and older should stop aspirin use for the prevention of cardiovascular disease.

Although October has long been celebrated as National Pharmacy Month, it is only in recent years that female pharmacists have been celebrated for their contributions to the profession and patient care.

Soliman is ecstatic to celebrate the 4th annual #WomenPharmacistDay and that the pharmacy landscape is continuing to recognize the contributions of women in the field.

The Pharmacy Times® Pharmacy Focus podcast provides the latest industry news and information, thought-leader insights, clinical updates, patient counseling tools, and innovative solutions for the everyday practice and business of pharmacy.

Cemiplimab-rwlc is being developed by Regeneron and Sanofi under a global collaboration agreement.
Menopause can happen in your 40s or 50s, but the average age is 51 years in the United States.