
There have been 15 events of deep vein thrombosis and 22 events of pulmonary embolism reported across the European Union and United Kingdom.

There have been 15 events of deep vein thrombosis and 22 events of pulmonary embolism reported across the European Union and United Kingdom.

The investigators observed a near normal age distribution among patients with long-term COVID-19 symptoms, including children.

Danielle DiBari, the chief pharmacy officer and senior vice president of business operations for NYC Health + Hospitals, discusses some of the challenges involved in coordinating vaccine distribution across New York’s public health system.
An ongoing phase 2 clinical study has begun to vaccinate patients with a modified version of Moderna’s COVID-19 vaccine.

A recent survey showed that there is a significant preference among Americans to receive COVID-19 vaccines from a trusted health care provider, such as a pharmacist or physician.

Chad Landmon, JD, chair of intellectual property and FDA practice groups at Axinn, Veltrop & Harkrider, discusses how the COVID-19 pandemic has impacted the FDA’s approval process.
The potential for some people to skip the second COVID-19 vaccine dose may make more doses available to others.
Pharmacists must first build trust with their communities in order to address vaccine hesitancy, according to Sally Arif, PharmD, BCPS.
In a recent statement, Walgreens announced that it has administered approximately 5 million COVID-19 vaccinations at long-term care facilities, in its stores, and through its vaccination clinics.
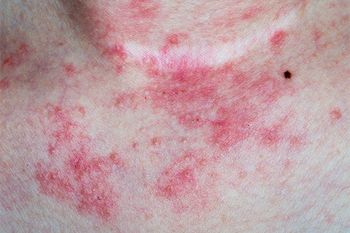
The study authors also mention that samples taken from skin biopsies confirmed their suspicion of a delayed allergic immune response that is commonly seen in drug reactions.

Pharmacy Times® interviewed Chad Landmon, JD, chair of intellectual property and FDA practice groups at Axinn, Veltrop & Harkrider, on how to approach discussing the FDA’s expedited EUA process for COVID-19 vaccines with patients with concerns about the process.
The FDA has issued an emergency use authorization (EUA) for a COVID-19 diagnostic test that can confirm recent or prior COVID-19 infection.

The investigators urged continued safety measures, such as social distancing and school closures, in order to prevent more COVID-19 variant-related deaths and hospitalizations.
In a recent interview with Pharmacy Times, Council Powell, PharmD, region director with CVS, said pharmacists and technicians are hard at work administering COVID-19 vaccines around the country.
The vaccine has been shown to induce mucosal immunity in the nasal cavity, which may be effective in blocking transmission of SARS-CoV-2.
Pharmacists can play an important role in educating the public about the safety of the Pfizer and Moderna COVID-19 vaccines.

In a CDC press briefing, experts said the vaccine’s single-dose approach will enable more mobile units and pop-up events to vaccinate hard-to-reach populations.
Should workers be required to receive immunizations to protect public well-being?

As Pharmacy Times® goes to press this month, there is a lot of encouraging news on the coronavirus 2019 (COVID-19) front.

Pharmacy Times® interviewed Nurisha Wade, MBA, and Farah Towfic, PharmD, MBA, of US Pharmacopeia, on how the release of the COVID-19 Vaccine Handling Toolkit may help pharmacists quickly and safely vaccinate.

Pharmacy Times® interviewed Micah Cost, PharmD, MS, who was recently selected as the new chief executive officer of PQA, on his plans for the future of the organization.

Vaccine hesitancy is a main driver for lower than desired vaccination rates and recent outbreaks of vaccine-preventable illnesses, such as measles, in the United States.

Pharmacy Times® interviewed Brian Nightengale, president of Good Neighbor Pharmacy, on the role of independent pharmacies in the country during the COVID-19 pandemic.
Pharmacies must be ready to store, handle, administer, and follow up per local, national, and state regulations.

In a recent letter sent to President Biden, ASHP and more than 50 other health systems and provider organizations requested that the president activate federal resources to establish mass COVID-19 vaccination sites.