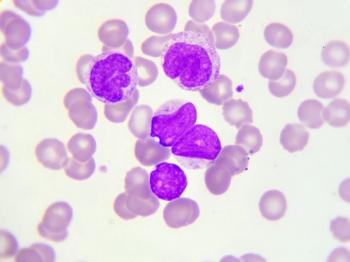
Patients with AML that has gone into remission following initial chemotherapy were found to remain in remission longer and have improved OS if they took a pill form of azacitidine as a maintenance treatment.


Patients with AML that has gone into remission following initial chemotherapy were found to remain in remission longer and have improved OS if they took a pill form of azacitidine as a maintenance treatment.
The study authors found marked improvements in rates of hazardous prescribing over the 12-month study period and the success of the intervention was sustained at the end.

Stroke is not among the covered diagnoses for cardiac rehab, despite many similar cardiovascular risk factors, according to the study.
Numerous therapeutic options are available for acute myeloid leukemia, but more research is needed to determine how best to use them.

This activity is supported by an independent medical education grant from Regeneron Pharmaceuticals, Inc.
The blood test measures levels of mitochondrial DNA, which is a unique type of DNA molecule that normally resides inside the energy factories of cells.
New treatment approach is designed to treat retracted blood clots, which form over extended periods of time and are especially dense.

Crizotinib is the first biomarker-driven therapy for relapsed or refractory anaplastic large cell lymphoma in children and young adults.

The FDA has granted an orphan drug designation to the BCMA-targeted trispecific T-cell activating recombinant protein construct as treatment for patients with multiple myeloma.
The current study provides characterization of a lipid-rich necrotic core, a dangerous type of coronary plaque made up of dead cells and cell debris that is prone to rupture, which can lead to a heart attack or stroke.

In patients with severe cardiac failure, the extreme emaciation of the body is a potential result and has been termed cardiac cachexia.
In a recent study at the National Institutes of Health, researchers consistently observed signs of damage on the brain scans of patients who died after contracting COVID-19.

Pralatrexate, a lymphoma drug, outperformed remdesivir in treating COVID-19 in a lab experiment.

Per Morten Sandset, MD, of the University of Oslo and Oslo University Hospital, discusses the treatment considerations necessary to properly manage a patient with congenital plasminogen deficiency after she became pregnant.

Per Morten Sandset, MD, of the University of Oslo and Oslo University Hospital, discusses the details of a patient who had a successful pregnancy despite documented infertility due to congenital plasminogen deficiency.

The biologics license application lisocabtagene maraleucel in adult patients with relapsed/refractory large B-cell lymphoma following at least 2 previous therapies continues to be under regulatory review by the FDA.

The research team designed TETi76 to replicate and amplify the effects of a natural molecule called 2-hydroxyglutarate (2HG), which inhibits the enzymatic activity of TET genes.

Per Morten Sandset, MD, of the University of Oslo and Oslo University Hospital, discusses the disease impact on the gynecologic system and how often patients become infertile due to this impact.

Per Morten Sandset, MD, of the University of Oslo and Oslo University Hospital, details the treatment options available for patients with congenital plasminogen deficiency.
More and newer treatment options mean that patients will benefit from various regimens and strategies.
Per Morten Sandset, MD, of the University of Oslo and Oslo University Hospital, describes common manifestations of congenital plasminogen deficiency and their impact on patient outcomes.
Per Morten Sandset, MD, of the University of Oslo and Oslo University Hospital, discusses why congenital plasminogen deficiency is often underdiagnosed and describes the causes and characteristics of the disorder.

Belantamab mafodotin-blmf (Blenrep; GlaxoSmithKline) is the first in its class anti-BCMA therapy to gain FDA approval.
Three monoclonal antibodies, 1 antibody-drug conjugate, and 1 nuclear export inhibitor have been approved by the FDA for relapsed/refractory (R/R) multiple myeloma in the past 4 years.

With the explosion of new therapies for cancer, the role of the oncology pharmacist continues to expand.