Gastrointestinal Health
Latest News
Latest Videos
CME Content
More News

Ustekinumab-stba (Steqeyma; Celltrion) is a subcutaneous treatment for adult and pediatric patients with plaque psoriasis arthritis, psoriatic arthritis, Crohn disease, and ulcerative colitis.
The risk of therapy duplication with OTC and prescription PPIs can be lowered with an innovative, multi-faceted, and intentional approach by pharmacy teams and prescribers.

The primary end point of clinical remission at week 26 was met, with 36.7% of individuals treated with RHB-104 achieving clinical remission.

An analysis of interaction checkers and product summaries revealed large differences in the consistency and reliability of providing accurate information on potential drug-drug interactions.

Study shows that orange peel extract may reduce risk of heart disease and adverse cardiovascular events.

In their concluding remarks, Arun Jesudian, MD; Ralph J. Riello, PharmD, BCPS, and Chas McCormick, RPh, MBA, summarize the key takeaways and highlight the persistent unmet needs in the management of hepatic encephalopathy.

Key opinion leaders highlight essential considerations for pharmacists, including medication costs, staying current with the latest advancements and clinical trials in hepatic encephalopathy (HE), and understanding the incidence of the disease.

Experts explore strategies for motivating treatment adherence and the importance of providers ensuring patients thoroughly understand their condition.

The primary endpoint of the study was clinical remission at week 52 with vedolizumab

Melatonin is linked to circadian rhythm, mitochondrial function, and neuroinflammation.

Individuals included in the study reported positive changes in fatigue, and disease activity displayed endoscopic and histologic remission.

Children with normal weight had different community relationships compared to children with overweight or obesity.
Repairing the intestinal epithelial barrier is paramount in the treatment of IBD.


Nearly 60% of patients who regained all the weight stayed in remission at 5 years post-surgery, according to study results.
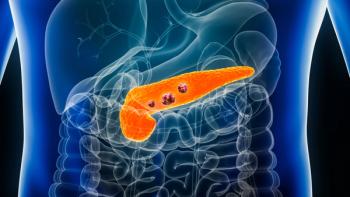
The approval of irinotecan liposome offers an improved first-line treatment option for individuals diagnosed with metastatic pancreatic adenocarcinoma.
Researchers will continue to research how lantibiotic’s antimicrobial effects can be harnessed for good.
The designation offers support in developing potential new medicines, treatment, and diagnosis to prevent rare conditions, like EoE.
Radioligands may be able to deliver radiation to advanced tumor cells anywhere in the body.

Jennifer Marsh, PharmD, BCCCP, discusses the current standard of care for hepatic encephalopathy, highlighting challenges in treatment, prescription drug coverage, transition of care, and outpatient treatment access.
There may be a pathophysiological basis for therapy relating to the to the gut-liver axis.
Findings from a recent murine model may translate to the human gut microbiome, but assessing chlorine’s effects on the gut microbiome remains complex.

Despite the insignificant differences in efficacy, biomarkers, therapeutic drug level, and ADAs, patients reported a significant number of nocebo effects.
Iberzaplstat was non-inferior to standard of care vancomycin, was safe and tolerable, and will advance into a phase 3 trial.
No significant demographic or phenotypic differences were found between HLA-DQA1*05 carriers versus non-carriers, in either treatment group.