Biosimilars
Latest News
Latest Videos
CME Content
More News

Although more research is needed in pediatric patients, biosimilars could improve treatment access and reduce costs.

Gaps in education about biosimilars, particularly among pharmacists, patients, and prescribers, are a significant barrier to wider adoption.

The approval in multiple indications is designed to improve access to effective, proven treatments for skeletal fractures, which can greatly reduce patient quality of life.

Insulin-aspart-szjj (Merilog) is the first rapid-acting insulin biosimilar product approved by the FDA.
If approved, HLX11 could offer a more cost-effective alternative to pertuzumab for the treatment of human epidermal growth factor receptor 2-positive breast cancer.

Tocilizumab-anoh is expected to increase competition, improve patient access, and deliver cost savings in the treatment of various inflammatory conditions.

The approval includes indications for rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and COVID-19.

This marks the first US biologic license application filing acceptance for a biosimilar candidate to golimumab.
Biosimilar use is not linked to an increased risk of treatment failure among pediatric patients with inflammatory bowel disease (IBD).

The US health care market faces transformative changes in 2025, as biosimilars, GLP-1s, and new pharmacy economics reshape how benefit managers balance innovation with affordability.

Successfully integrating biosimilars into practices requires careful planning and execution, as they impact virtually all aspects of a practice.
Serplulimab plus HLX04 demonstrates a favorable safety profile in patients with advanced hepatocellular carcinoma.

Adherence to thiopurine medications after 6 months of use was 87.9% under direct pharmacist care but 65.7% under general practitioner guidance.

Adoption barriers include insurance coverage, patient and provider education, and regulatory hurdles that require aligned initiatives across stakeholders.

The evolving regulatory policies around biosimilar interchangeability is crucial for driving successful biosimilar adoption in the marketplace.

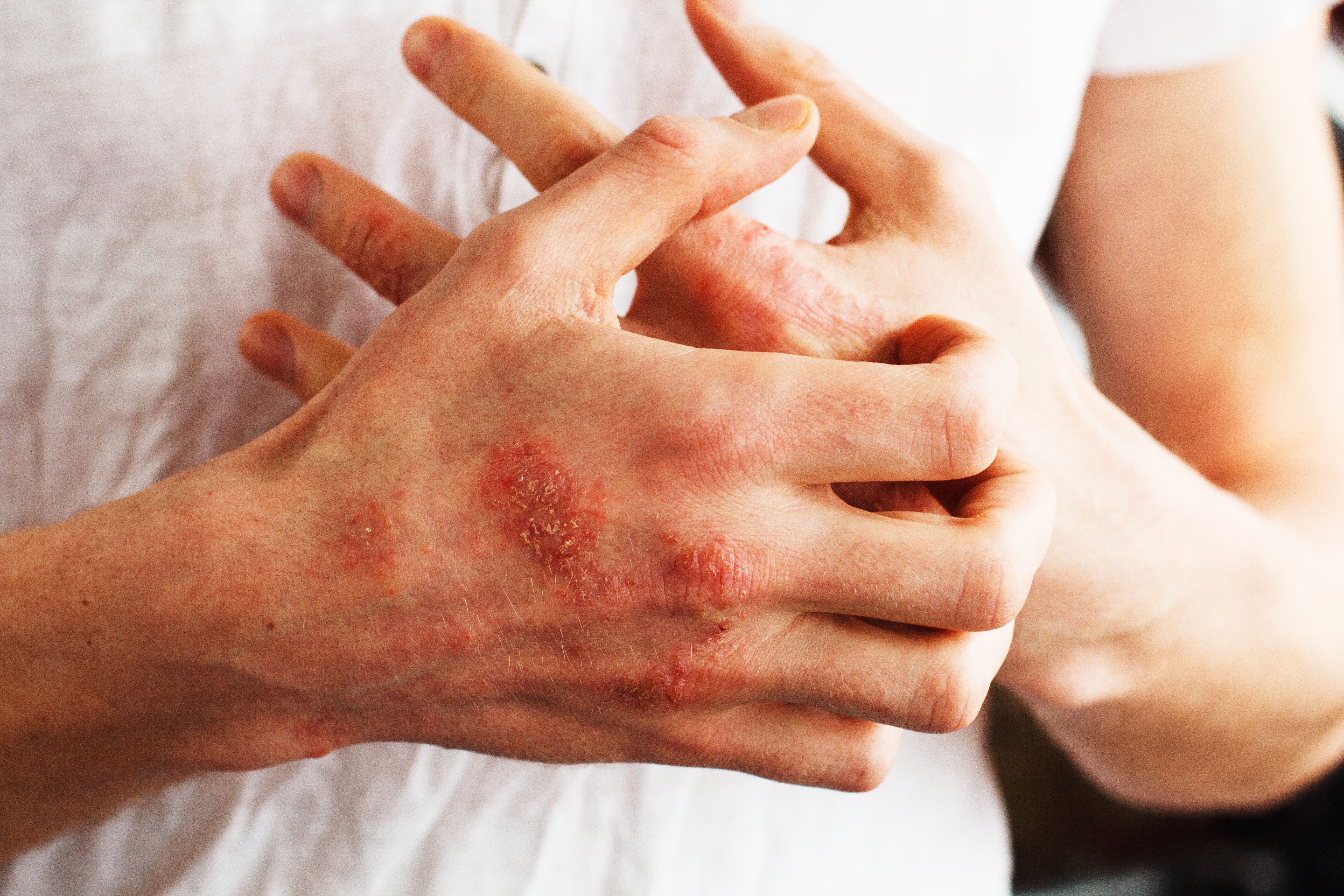
Ustekinumab-stba (Steqeyma; Celltrion) is a subcutaneous treatment for adult and pediatric patients with plaque psoriasis arthritis, psoriatic arthritis, Crohn disease, and ulcerative colitis.

The tool is designed to address the nocebo effect, which was a concern of prescribers and other health care professionals.

ASHP Midyear: Pharmacists Play Key Role in Addressing Concerns and Fostering Adoption of Biosimilars
Pharmacists can help drive the successful adoption of biosimilars by developing educational programs to address provider and patient concerns.

Investigators presented additional data from a clinical trial at the American College of Rheumatology Convergence, demonstrating similar safety for postmenopausal women with osteoporosis.

The biosimilar is approved for the treatment of Crohn disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis.

However, the monthly expenditure was not significantly associated with the financial incentives.
There were 2 main types of switches, including switching to rituximab subcutaneous and switching among different intravenous rituximab treatments.
Ocrelizumab (Ocrevus; Genentech) is used for the management and treatment of multiple sclerosis.

Pegfilgrastim (Neulasta; Amgen) treats neutropenia that is caused by cancer medications and helps the bone marrow to create new white blood cells.