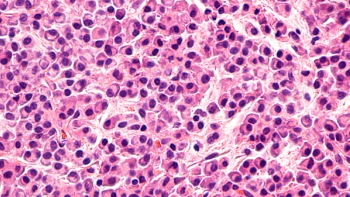
Hagop Kantarjian, MD, of the University of Texas MD Anderson Cancer Center, discusses what the interim analysis of the OPTIC trial demonstrated in terms of the safety and arterial occlusive events profiles with response-based ponatinib dosing regimens.