The agent demonstrated promising tolerability and manageable safety.

The agent demonstrated promising tolerability and manageable safety.
The 12.5-mg dose tablet enhances hypertension treatment options and reduces adverse effects for patients.

The agent targets B7-H3, a commonly overexpressed protein in diffuse intrinsic pontine glioma (DIPG).
Currently, the treatment is undergoing evaluation in a 3-part clinical trial to determine its safety, efficacy, and pharmacokinetics in patients with recurrent advanced/metastatic cancers.

In clinical trials, the dihydroergotamine (DHE) nasal powder had favorable safety profiles and rapid pain relief in patients with migraine with or without aura.

Nipocalimab the first and only FcRn blocker approved in anti-acetylcholine receptor– or anti-muscle-specific kinase adults and children with generalized myasthenia gravis.

FDA approves Zevaskyn, the first gene therapy for RDEB, offering hope for effective wound healing and pain relief in patients.

A 15-mg dose with a corticosteroid taper regimen was superior to placebo in sustained remission from week 12 to 52.
NS-229 is a Janus kinase 1 (JAK1) inhibitor being investigated in eosinophilic granulomatosis with polyangiitis, a rare autoimmune condition that can cause severe allergic and asthmatic symptoms through inflammation of nerve cells.
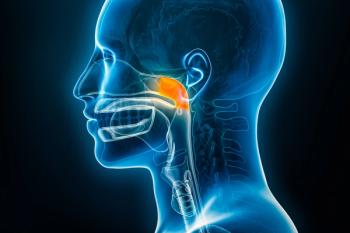
The treatment received indications as a monotherapy and in combination regimen with carboplatin or cisplatin and gemcitabine.

The in vivo gene editing program is designed to eliminate cccDNA and inactive integrated hepatitis B DNA.


An oral dose of 0.6 mg per 5 mL of colchicine could aid individuals who have trouble with swallowing pills.
The breakthrough designation is supported by clinical data from ongoing phase 1/2 trials.
Multiple phase 3 clinical trials demonstrated dupilumab’s (Dupixent; Regeneron, Sanofi) effectiveness in reducing hives and itch in patients with chronic spontaneous urticaria compared with placebo.
Some patients with aplastic anemia treated with CK0801 achieved durable transfusion independence lasting over 3 years.

The safety profile of diazepam nasal spray among pediatric patients was consistent with older patients, supporting its use in this age group.

The designation was supported based on data from the randomized, double-blind, active-controlled phase 3 interchangeability study.
The new indication expands administration methods for the combination of efgartigimod alfa and hyaluronidase-qvfc, which are already approved for the treatment of some immune-related conditions.

Patients with unresectable or metastatic hepatocellular carcinoma (HCC) receiving this regimen had favorable median overall survival (23.7 months) compared with standard of care (20.6 months).

This approval marks bevacizumab-nwgd as the sixth reference product to bevacizumab (Avastin).
The approval is for those with metastatic disease or in whom surgical resection is likely to result in severe morbidity, have no satisfactory alternative treatments, or have progressed following treatment.
Data show that the system is also the most accurate continuous glucose monitor (CGM).

The approval was based on efficacy and safety data from a randomized, 3-arm, open-label phase 3 CHECKMATE-8HW trial.
The new designations span both warm autoimmune hemolytic anemia and IgG4-related disease, 2 rare, immune-mediated conditions that burden patients due to a lack of available treatment options.
BIIB080 is designed to target microtubule-associated protein tau mRNA, aimed to reduce the production of tau protein.

This action makes inebilizumab the first and only FDA-approved treatment for adults with immunoglobulin G4-related disease (IgG4-RD).
No manufacturing or safety issues were identified, but the FDA stated the new drug application did not demonstrate efficacy in adequate and well-controlled studies.
Positive results from an interim analysis of the phase 3 ALIGN study bolstered atrasentan towards receiving accelerated approval for patients with immunoglobulin A nephropathy, a rare condition that can cause kidney failure.
The Auto-Pure 2400 system combines liquid handling and magnetic cell isolation for efficient latent tuberculosis testing.