The FDA approved a 2.9-mg/0.71-mg dosage strength of Orexo's sublingual buprenorphine/naloxone (Zubsolv).

The FDA approved a 2.9-mg/0.71-mg dosage strength of Orexo's sublingual buprenorphine/naloxone (Zubsolv).

The FDA today approved eluxadoline and rifaximin to treat irritable bowel syndrome with diarrhea.


Chronic pain patients using prescription opioids in conjunction with medical marijuana are not at increased risk for substance abuse.

Regular trips to the emergency department could be a red flag for deadly prescription drug overdose.

Despite advances in pain management and palliative care, intractable cancer pain remains a significant clinical, social, and financial burden in treating the oncologic population.

Deaths related to oxycodone overdose dropped 25% in Florida after the state adopted a Prescription Drug Monitoring Program to track controlled substance dispensing.

The FDA today expanded the indication of Allergan's onabotulinumtoxinA (Botox), allowing the drug to be used for the treatment of upper limb spasticity in adults.

In addition to relieving pain, acetaminophen may cause patients to experience less intense emotional responses.

The FDA is warning pharmacists that pets are at risk of death when exposed to topical pain medications containing flurbiprofen.

Over my past 3 decades of pharmacy practice, acetaminophen has been one of my most recommended OTC treatments.

A pharmacist who posted derogatory comments about a customer on social media is now being investigated by the National Health Service.

Pharmacists are positioned to educate and monitor older patients who are discharged on these medications.
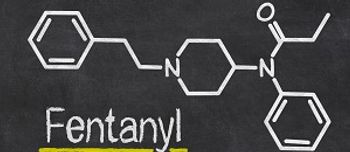
In response to a recent resurgence in fentanyl abuse, the DEA issued a nationwide alert about the dangers of the drug, describing it as a threat to health and public safety.
From optimizing inhaler technique in patients with chronic obstructive pulmonary disease, to catching red flags when dispensing controlled substances to patients with chronic pain, pharmacists can improve patient outcomes in an evolving treatment landscape.

Mylan Inc. has introduced 3 generic versions of existing drugs to the US market, including the first and only available intermediate dosages of transdermal fentanyl.

More than 300 pharmacy students, technicians, and pharmacists helped raise nearly $6500 at the 2015 Mid-Year Conference of the Pennsylvania Pharmacists Association.

I distinctly remember feeling surprised when the FDA approved the first single entity and extended-release hydrocodone pain medication.

Are pharmacists fully aware of the warnings associated with the medications they dispense?
Most people hear "hospice" and think "cancer," but many end-stage chronic obstructive pulmonary disease patients also receive hospice care when their caregivers can no longer provide the medical attention they need.

Dispensing higher opioid doses to chronic pain patients could trigger depression.
Narcotics are not recommended for managing chronic pain in children with inflammatory bowel disease, due to gastrointestinal side effects and potential dependence. Nevertheless, researchers have uncovered that long-term narcotic use is more than twice as prevalent in pediatric IBD patients compared with the general population.

Hospira is voluntarily recalling 63 lots of its ketorolac tromethamine injection following a confirmed report of calcium-ketorolac crystals floating in glass vials of the product.

The FDA has accepted for review a New Drug Application for Inspirion Delivery Technologies' investigational opioid analgesic, MorphaBond ER, an abuse-deterrent formulation of extended-release morphine.
A primary goal of managing atrial fibrillation is relief of symptoms that include dizziness, fatigue, palpitations, shortness of breath, and chest pain. Yet, it is symptomless in up to one-third of patients.