Immunization
Latest News
Latest Videos

CME Content
More News
Pharmacists play a critical role in educating patients on RSV prevention, how to recognize early symptoms, and when to seek medical help.

Pharmacists can play a crucial role in educating parents and helping remind them about the childhood vaccine schedule, which can be quite complex.
The pharmacy is no longer considered to offer full service (or to be financially sustainable) without a robust vaccination practice.
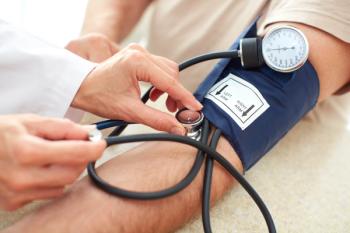
Despite study findings showing that 1 in 5 patients hospitalized for COVID-19 developed persistent high blood pressure, many had underlying risk factors.
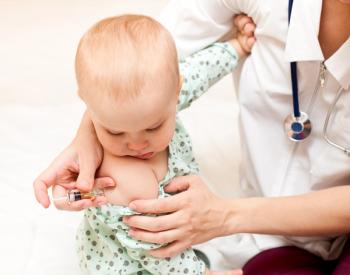
Nirsevimab (Beyfortus) is the first respiratory syncytial virus (RSV) preventative drug to be approved by the FDA, and can protect infants through their first RSV season for up to 5 months.

Investigators from Griffith University stated that technology has helped the development of vaccines that are safe to use and induce strong immune responses against Strep A.
This technique may be applied to transdermal applications, such as shot-free vaccines.

UB-311 has shown to be well-tolerated with a durable antibody response in a previous phase 1 trial, and new results from a phase 2a study support its continued development.
The approach allows investigators to assess post-vaccine response in immunocompromised patients who have received antibody prophylaxis.
The findings of the study demonstrate the need for preventive interventions for all infants in order to reduce the burden of severe illness from respiratory syncytial virus lower respiratory tract infections.
A COVID-19 booster dose increased both maternal and cord blood binding and neutralizing antibody levels against virus strains, including Omicron BA.1.

Pharmacies brace for a double wave of DIR fees while paddling against profit struggles. Enter Pharmacy AI: a transformative vehicle to navigate stormy waters, enhance efficiency, and prioritize patient care.
Gretchen Garofoli, PharmD, an associate professor at the West Virginia School of Pharmacy, discusses the current recommendations for COVID-19, respiratory syncytial virus (RSV), and influenza vaccines and vaccine hesitancy.
Approximately 57% of individuals who were shown a flowchart of the FDA vaccine approval process were very or somewhat likely to recommend a respiratory syncytial virus vaccine to a pregnant family member or friend compared to only 40% of those who were not shown the process.

A bit of preparation can set children up for success and reduce the stress of this busy time.
Compared to the BNT162b2 vaccine, the mRNA-1273 vaccine was associated with a lower risk of adverse events, which investigators speculate was due to interrelated safety and efficacy.
CDC Advisory Committee on Immunization Practices Recommends Nirsevimab-alip to Prevent RSV in Infants
CDC Advisory Committee on Immunization Practices voted 10 to 0 to recommend the routine use of nirsevimab-alip (Beyfortus; Sanofi and AstraZeneca) for the prevention of respiratory syncytial virus lower respiratory tract disease in newborns and infants.

FDA Approves Expanded Indication of Ebola Zaire Vaccine for Individuals 12 Years of Age and Older
Ervebo was found to be 100% effective after vaccination in preventing the onset of Ebola virus symptoms after more than 10 days.
Study shows maternal vaccination with Pfizer’s hexavalent capsular polysaccharide conjugate vaccine may protect infants against Group B Streptococcus.
If approved, the V116 vaccine would be the first pneumococcal conjugate vaccine specifically designed for adults.

Community-based pharmacy services are reaching new heights as pharmacists and pharmacy technicians are able to have a larger impact on patient care than ever before.
The prolonged lack of viral exposure has likely increased the pool of children and adults vulnerable to respiratory syncytial virus, but questions remain.
With the end of the national public health emergency for COVID-19 in May 2023, what can be expected as we look ahead to the 2023-2024 influenza season and beyond?

The approval marks the first monoclonal antibody approved to protect all infants through their first respiratory syncytial virus season.
Every pharmacist can make a difference in patients’ lives by taking the time to understand and fulfill the community’s social and cultural needs.