
But 3 main challenges stand in the way of their adoption: patent litigation, patient and physician concerns, and rebates and reimbursements.

But 3 main challenges stand in the way of their adoption: patent litigation, patient and physician concerns, and rebates and reimbursements.
More aggressive competition and uptake could cause larger cuts, estimated at up to $124.5 billion between 2021 and 2025, according to the results of a RAND corporation analysis.

Insulin glargine-yfgn (Semglee; Viatris) presents the first opportunity for retail pharmacists to dispense a biosimilar to patients.
In the case reviewed, the pharmacy department created an interdisciplinary Biosimilar Task Force responsible for making decisions relating to the adoption of biosimilar therapies.

Dissimilarities include acceptance by third-party payers, applications, dispensation, prices, and use.
An anti-vascular endothelial growth factor therapy, ranibizumab prevents vision loss in patients with retinal vascular disorders, which can cause irreversible blindness or visual impairments in adults.

Cheryl Larson, president and CEO of Midwest Business Group on Health, discusses current efforts to manage drug spend and the high cost of specialty drugs on the market.
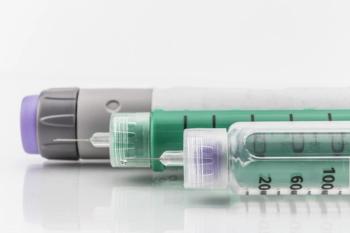
The introduction of a biosimilar insulin could result in major shifts in the accessibility and affordability of insulin for all patients.
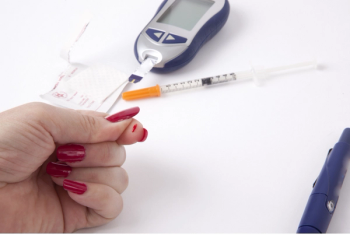
The availability of insulin biosimilars can bring high-quality, lower cost treatment options for pharmacists, physicians, payers, and patients alike.
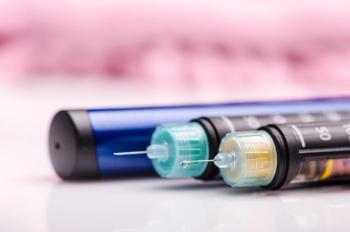
Insulin glargine-yfgn is the first interchangeable biosimilar product for the treatment of diabetes approved in the United States, which can provide patients with alternative, equally effective options for treating diabetes that could be more cost effective.

This week on Pharmacy Times, there are a number of important topics that will be covered and posted throughout the week.

The pipeline for biosimilar products in the United States includes at least 26 candidates in phase 3 trials for 13 reference therapies.
Bhavesh Shah, RPh, BCOP; Brandon Dyson, PharmD, BCOP, BCPS; Ryan Haumschild, PharmD, MS, MBA; and Tim Peterson, PharmD, BCOP, provide advice and strategies for adopting biosimilars into clinical practice.

The impact of the COVID-19 pandemic on the rate of biosimilar adoption in clinical practice, updates to NCCN guideline recommendations, and financial implications associated with the utilization of biosimilars.

The influence of provider buy-in for getting a biosimilar onto formulary and the role of providers in the incorporation of biosimilars are discussed.

Overcoming barriers for payer coverage of biosimilars as well as considerations for carrying costs are discussed.
Brandon Dyson, PharmD, BCOP, BCPS, shares his approach to switching a patient already on biologic therapy to a biosimilar, and panelists discuss the importance of education regarding the extrapolation of indications for biosimilars.

A discussion on the extrapolation of European data to aid in P&T (Pharmacy and Therapeutics) committee decisions to adopt the use of biosimilars.

Experts discuss managing biosimilar adoption in various clinical practice settings, working through the P&T (Pharmacy and Therapeutics) approval process, and the importance of relying on a shared-decision making framework.
Ryan Haumschild, PharmD, MS, MBA, provides insight on biosimilar adoption both in oncology and non-oncology practice settings.

The impact on biosimilar utilization in clinical practice caused by secondary patents of biologics and skinny labels of biosimilars is discussed.
Tim Peterson, PharmD, BCOP, leads the discussion on immunogenicity testing and lot-to-lot variability, including the potential impact on the safety and efficacy of reference biologics and their biosimilars.

A discussion on what it means for a biosimilar to have “interchangeability” status and its potential impact on biosimilar adoption in clinical practice.

Ryan Haumschild, PharmD, MS, MBA, defines biosimilars in oncology and what distinguishes them from a reference biologic.

Pharmacy Times® interviewed Chad Landmon, JD, of Axinn, Veltrop & Harkrider, on the arguments presented to the Supreme Court on whether a legislative change to the Affordable Care Act makes the law unconstitutional.