Amy Duong, PharmD, BCACP, highlights the importance of patient counseling and addressing logistical challenges in treating patients with chronic inflammatory demyelinating neuropathy (CIDP).

Amy Duong, PharmD, BCACP, highlights the importance of patient counseling and addressing logistical challenges in treating patients with chronic inflammatory demyelinating neuropathy (CIDP).

Its approval was based on data from a 3-year trial that enrolled patients with alkaptonuria.

In the first half of 2025, the FDA approved 7 novel drugs for oncologic conditions, including for non-small cell lung cancer; ovarian cancer; and metastatic, hormone receptor-positive, human epidermal growth factor 2-negative breast cancer.
The authors note that the combinatorial principal component analysis (cPCA) may be effective in other complex diseases outside of chronic kidney disease (CKD).
This abstract will be presented at the Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025.

Explore the evolving role of pharmacists in patient care, emphasizing personalized services, community health, and innovative solutions for better health outcomes.

Amy Duong, PharmD, BCACP, explains pharmacists' critical role in promoting chronic inflammatory demyelinating neuropathy (CIDP) treatment adherence and staying informed about new therapeutic developments.

Pharmacists are redefining health care by bridging clinical and economic evidence, making a real difference in patient outcomes through health economics and outcomes research (HEOR).
Patients with chronic kidney disease (CKD) and type 2 diabetes receiving both treatments had greater reductions in urinary albumin-to-creatinine ratio.

Amy Duong, PharmD, BCACP, highlights pharmacists' essential responsibilities in chronic inflammatory demyelinating polyneuropathy (CIDP) patient care and immunoglobulin therapy management.

Pharmacists play a critical role in managing complex pericarditis cases.

Pharmacists lead the charge in implementing advanced therapeutics, navigating complex challenges and shaping the future of health care innovation.
Rilonacept offers a targeted approach to treating recurrent pericarditis by blocking IL-1 inflammatory pathways.

Subcutaneous immunoglobulin was found to elicit fewer viral infections compared with other routes of immunoglobulin replacement therapy, including intravenous immunoglobulin.
The investigators suggested that blocking VPS72’s interactions can be a foundation for future therapeutic interventions.

Subcutaneous immunoglobulin (SCIg) is a hopeful alternative to intravenous immunoglobulin in patients with inflammatory myositis due to a lack of adverse effects and fewer financial constraints.

New findings show LYR-210's potential to significantly alleviate chronic rhinosinusitis symptoms, offering hope for patients with nasal inflammation and polyps.

New treatments for metabolic dysfunction-associated steatohepatitis (MASH)–related cirrhosis address unique challenges in drug development.

Chip Hailey, a 74-year-old pharmacist with hemophilia B, shares how gene therapy has liberated him from decades of challenging treatment and restored his independence.
IVIG treatment significantly boosts platelet counts in pregnant people with thrombocytopenia, revealing key predictors for effective response and optimizing clinical decisions.

The FDA grants fast track designation to TEV-53408, a promising treatment for celiac disease designed to address gluten intolerance and improve patient outcomes.
Researchers explore cellular senescence's role in kidney fibrosis, highlighting potential therapies to combat chronic kidney disease progression.
The test offers health care professionals a simple and efficient method of identifying patients with liver fibrosis of varying severity.
The investigators suggest that early detection and treatment of chronic kidney disease (CKD) in childhood cancer survivors may decrease late complications and mortality.
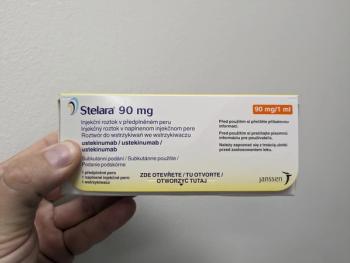
A new biosimilar to ustekinumab (Stelara) has been approved by the FDA, further expanding treatment options for patients with immune conditions such as plaque psoriasis and psoriatic arthritis.

Pharmacists have an evolving role in biotechnology.
The small molecule effectively blocks cell death effector proteins, potentially rescuing cells from degeneration.

Pharmacists can make the process easier and more convenient for patients.

The process teaches resilience, adaptability, and self-awareness.