Individuals received 4 daily short messages that reminding them to participate in self-care, active engagement, and early intervention for the first 30 days of the study.

Individuals received 4 daily short messages that reminding them to participate in self-care, active engagement, and early intervention for the first 30 days of the study.
Investigators aim to find a substitution for incomplete Freund adjuvant, which is not suitable for use in humans but shows a strong response to antigen-specific systemic immune responses.
Preclinical data showed that LP-184 shrunk tumors in vivo by more than 90% in just 8 weeks.
Language gaps resulted in barriers and underdiagnosing in children with common allergies, limiting support for these patients and their families.

This approval makes birch triterpenes the first treatment for wounds associated with junctional epidermolysis bullosa to be approved by the FDA.
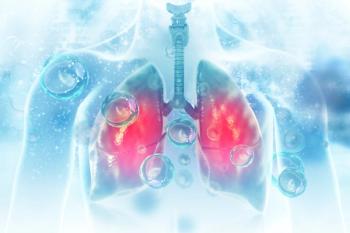
If approved, V116 would offer significant improvements in safety and effectiveness for the prevention of pneumococcal disease.
Amiodarone also provides pan-variant protection against toxins, a potentially growing area of research in C. difficile infection treatment.

The study authors note that interventions to reverse frailty, such as screening and assessments to identify issues, can help in the management of COPD and asthma and lead to better prognoses.
Previously, garadacimab was granted an orphan drug designation as a therapy for hereditary angioedema by both the FDA and EMA.
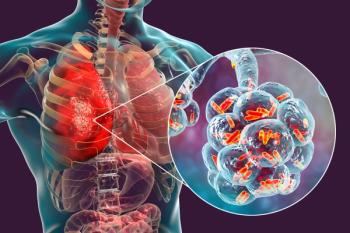
Less than 1% of individuals developed Clostridioides difficile infection (CDI), but for those who previously had a CDI the year before, 12% developed a new infection.

Treatment options can be limited for this patient population.

Fran Gregory, PharmD, MBA, vice president of emerging therapies at Cardinal Health, discusses the dynamic landscape of the biosimilars market, providing key insights into its current status and future projections.

Using information from clinical studies and her experiences working in the field, Stephanie Kirk describes treatment options that are on the horizon, and how removing stigma is key in HIV care.
In the study, a new policy empowered unit-based pharmacists to evaluate the patient’s needs and, when safe, switch from a PPI to an H2 blocker.
The approval marks the first for an alternative to platinum-containing chemotherapy.
The study, which consists of 3 trials, will continue with oral PrEP regimens; however, the vaccination trials have stopped due to the unlikely chance of the vaccine showing efficacy.

Stress usually occurs as a byproduct of workplace powerlessness because feelings of powerlessness at work prevent us from being the kind of human beings we need to be.
Belzutifan (Welireg; Merck) demonstrated superior progression-free survival compared to everolimus in advanced renal cell carcinoma.

This month's Pharmacy Times Condition Watch features obesity and type 2 diabetes.

Investigators also found that the results demonstrated lower death and hospitalization rates for those with advanced heart failure treated with cell therapy.

Hospice care is only a part of palliative care services.
Feldman, who previously had C. diff, urges individuals to begin having conversations to help remove the stigma of the disease and raise awareness of its prevalence.

This is the first FDA approval for a therapy intended to reduce the risk of relapse in patients with high-risk neuroblastoma.

Feldman details her experience with C. diff, how the COVID-19 pandemic slowed the timeline of care, and how her surgical history and the use of antibiotics played into the disease’s onset.

Collaboration and knowledge sharing amongst rare disease researchers can be difficult, as these researchers are often spread out throughout the world.

In the pharmacy, time is the ultimate value-add.

Clinical trial results show the immunogenicity, tolerability, and safety of V116 compared to a pneumococcal 20-valent conjugate vaccine for adults who had not previously received a pneumococcal vaccine.
Bivigam was initially approved by the FDA in May 2019 for the treatment of primary humoral immunodeficiency, including a group of genetic disorders.

In the phase 3 SURMOUNT-4 trial, investigators included individuals with at least 1 weight-related complication, including hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease.

The study authors note that low skin cancer screening (SCS) participation, the participants’ better health than nonparticipants, and examination biases may be contributing factors to the insignificant results.