
Pharmacists can educate patients on current clinical evidence of cannabis’s therapeutic benefit, as well as cannabis’s complexities, risks, and potential adverse effects.

Pharmacists can educate patients on current clinical evidence of cannabis’s therapeutic benefit, as well as cannabis’s complexities, risks, and potential adverse effects.
Positive data were presented at the 13th Meeting of the International Society of Pneumonia and Pneumococcal Disease in Cape Town, South Africa.

Identifying the best uses for these tools, whether in patient education, data collection, improving efficiencies, or other uses, could significantly improve the future of pharmacy.
When considering community-acquired pneumonia alone, the PCR test identified 113 bacterial detections compared with 57 for the standard of care test.
The data comes from 2 phase 4 trials, ENABLE and EMPOWER, which evaluated lanadelumab and the outcomes of treatment in adolescents with hereditary angioedema.

As data continue to develop in this arena, β-lactam therapeutic drug monitoring is likely to become more routine, as it has with drugs like vancomycin and the aminoglycosides.

Tune into this episode of “Public Health Matters” for valuable insights into the intersection of entertainment, health care, and child advocacy, highlighting the importance of creating supportive and interactive environments for pediatric patients.
Methods of treating cervical intraepithelial neoplasia are limited and invasive. The need for better treatments is clear; however, there are several promising therapies under development.

Celebrities, local advocates, patients, and pharmacists can effectively promote public health initiatives and raise disease awareness by authentically leveraging trusted relationships.

Findings show that magnetic resonance imaging and lumbar puncture are not always needed when managing complications of CAR T-cell therapies, but may be beneficial in certain cases.

Regardless, hygiene practices and prevention measures for SARS-CoV-2 should be strongly practiced to prevent all respiratory viruses.

Nazia Somani Babul, PharmD, BCACP, discussed her presentation about nontraditional pharmacy models, and particularly a model at her facility focused on comprehensive pain management counseling.

The approval comes after positive results from the PhALLCON study, however, further research is needed to confirm immature event-free survival findings.

The oral medication activates an “anti-hunger” molecule which may prevent people from overeating.

To date, this is the only targeted therapy indicated for the treatment of generalized pustular psoriasis.

This approval makes atidarsagene autotemcel the only therapy to be approved in the US for early-symptomatic early juvenile metachromatic leukodystrophy.

Team-based care models improve continuity of care and accessibility for patients.

The agency will focus on collaboration to protect public health; advancing regulatory approaches; developing standards, guidelines, and best practices; and supporting research that evaluates and monitors AI performance.

Pharmacists at this clinic work with patients to improve medication education, affordability, and access to care.
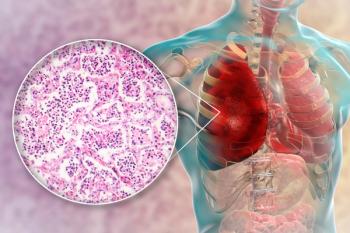
High bacterial load in cerebrospinal fluid was associated with unfavorable outcomes and death for adults with pneumococcal meningitis.

The FDA-approved topical and oral agent is currently indicated for adult patients with patterned alopecia.

Mila Felder, MD, FACEP, discusses Advocate Aurora Health’s approach to addressing burnout among health care professionals in health care systems.
Chimeric antigen receptor T-cell therapies were associated with higher incidences, grades of severity, and longer duration of cytokine release syndrome compared with bispecific antibodies.

In a phase 1/2 clinical trial, lisocabtagene maraleucel helped patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) achieve complete response rates.

Compared to placebo, risankizumab significantly reduced symptom severity for patients with moderate to severe disease.
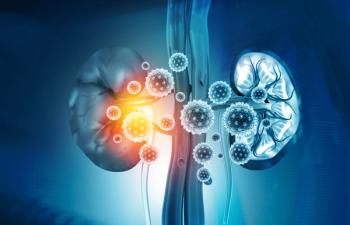
The drug is the first FDA-approved agent for treating hepatorenal syndrome.

Amina Abubakar, PharmD, AAHIVP, discussed her work throughout Africa and particularly in Kenya.

The platform is a first-of-its-kind therapeutic approach to promote remyelination among individuals with relapsing/remitting multiple sclerosis.

Results from the phase 3 RATIONALE 302 trial showed tislelizumab-jsgr prolonged survival compared to chemotherapy in patients who received prior systemic treatment.

The accelerated approval of resmetirom, a once-daily, oral thyroid hormone receptor-β agonist, is the first treatment available for patients with this disease.