
Relative risk calculations show that vaccinated individuals infected with the first Omicron subvariants of COVID-19 could have protection that lasts nearly 3 seasons.

Relative risk calculations show that vaccinated individuals infected with the first Omicron subvariants of COVID-19 could have protection that lasts nearly 3 seasons.

There have been more than 80,000 monkeypox cases reported worldwide.

Mothers, in particular, benefit when they are given longer leaves and policies that offer incentives or wage replacement, analysis indicates.

Lecanemab (Lequembi) approved to treat mild cognitive impairment or mild dementia associated with Alzheimer disease.
Pharmacists need to ensure anticoagulants are held appropriately before and after surgery to prevent complications from for CIED infections.

Ublituximab-xiiy from TG Therapeutics Inc is the first anti-CD20 monoclonal antibody approved for individuals with RMS that is administered as a 1-hour infusion after the starting dose.

The findings show promising clinical activity and a favorable tolerability profile in the treatment of KRASG12C-mutated advanced colorectal cancer.

MenABCWY combines components of 2 vaccines, which help protect against serogroups that cause most cases of the invasive disease.
A better understanding of the pathogenesis of monoclonal gammopathy of clinical significance would help guide clinicians treating the complex condition.

This technology could facilitate equal access to value-based pathways in oncology care and optimize the individual treatment approach.

The novel antibody will receive expedited review, according to the FDA, and already has market authorization in the European Union.
An overview of current theses and research surrounding the pathogenesis, presentation, and treatment of COVID-19 in children.
The opportunity to provide non-invasive colorectal cancer screening and education to patients is undoubtedly a role that pharmacists can fill.
The protein, called suPAR, has long been known to be a biomarker for poor outcomes and disease progression in both kidney disease and cardiovascular disease.

Big pharma companies have become more measured in their risk taking, with mergers and acquisitions gradually giving way to joint ventures and partnerships.

This week's episode features Tom Frieden, MPH, MD, who discusses his background and influence on public health and national policy.

Judy Neville speaks with pharmacy technicians about the impact of pharmacy technician as vaccinators.
A study showed that the risk of developing venous thromboembolism is worse for perimenopausal women with diabetes.

Children who experienced COVID-19-induced multisystem inflammatory syndrome did not experience severe adverse reactions following COVID-19 vaccination.
The FDA granted fidanacogene elaparvovec breakthrough, regenerative medicines advance therapy, and orphan drug designations.

The dual-action cell therapy is designed to eliminate established tumors, train the immune system to eradicate a primary tumor, and prevent recurrence.
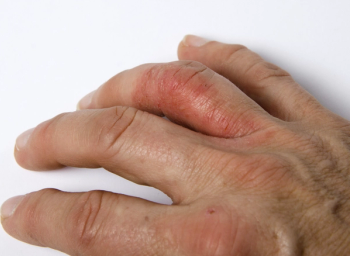
Greater prevalence of hand eczema among health care professionals working in COVID-19 units linked to increased hand sanitation habits.

The changes will almost certainly widen access for patients seeking medicated abortions in states without legal obstacles.
Biomarkers are commonly used by physicians to assist in the earlier diagnosis of some of the most common fungal diseases.

Embracing these new standards is the right thing to do for patient safety.

Some US pharmacies are experiencing medication shortages as a result of COVID-19, influenza, and respiratory syncytial virus cases.
Study finds a strong decline in hepatitis C incidence in the years immediately following broad access to direct-acting antiviral drugs.

The subcutaneous formulation could enhance treatment options by providing high consistency in drug exposure and a convenient administration method.

In addition to the physical symptoms of long COVID, patients have reported vocal, verbal, and cognitive issues that disrupt their ability to communicate.

Patient-centric models of medication delivery that provide cost, transparency, convenience, and accessibility solutions are vital for patients to successfully navigate the current health care system.