
According to a press release from AbbVie, Vuity is the first and only FDA-approved eye drop to treat this condition, which affects nearly half of the US adult population.

According to a press release from AbbVie, Vuity is the first and only FDA-approved eye drop to treat this condition, which affects nearly half of the US adult population.
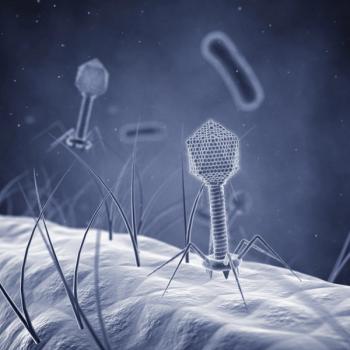
The study also found that certain E. coli cells in these microenvironments were able to survive phage treatment without acquiring genetic resistance.
The investigators said this study is one of the first to measure the impairment and impact of post-acute COVID syndrome on patients.
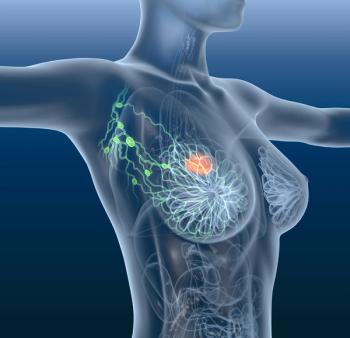
The study was designed to evaluate elacestrant as a monotherapy compared to the standard of care for the treatment of estrogen receptor-positive/human epidermal growth factor receptor 2 advanced or metastatic breast cancer.

This developing technology offers opportunities for decentralized record-keeping and tracking of transactions, as well as increased overall efficiency and the removal of unnecessary intermediaries.

The investigators evaluated and compared the immune responses induced by the 3 vaccines over the course of an 8-month follow-up period.
According to the investigators, these new data support ofatumumab as a first-choice treatment for adult patients with RMS, including those who are newly diagnosed.

Dissimilarities include acceptance by third-party payers, applications, dispensation, prices, and use.
The multi-institutional clinic trial also concluded that there was no negative impact to quality of life for these patients.
The investigators said that the trial data showed potentially clinically meaningful improvements in both progression-free survival and overall survival in pre-specified subgroups of patients based on the baseline inflammatory biomarker, hs-CRP, as well as other biomarker-defined subgroups.

In accordance with the new guidance, individuals may choose to receive a dose different from the one they received for their initial series.

According to the investigators, Susvimo is the first and only FDA-approved treatment for wet AMD that offers as few as 2 treatments per year.

Officials with the FDA have approved Roche’s VENTANA PD-L1 (SP263) Assay as a diagnostic test to evaluate patients with non-small cell lung cancer (NSCLC) who are eligible to receive atezolizumab (Tecentriq, Genentech).

The investigators found that the rate of preterm birth in pregnant women who tested positive for SARS-CoV-2 was a function of the severity of infection.

While Bitcoin and Ethereum garner most of the attention in the cryptocurrency space, there are a number of opportunities present for implementing blockchain into global health technology, with perhaps one of the most intuitive being its potential as a platform for health records.

TAK1 restricts receptor-interacting serine/threonine-protein kinase 1 dependent cell death, and targeting TAK1 with inhibitors shifts cells from pro-survival programs to cell death, according to the study.
New legislation is set to ensure the availability of home infusions for certain Medicare patients.

Officials with the FDA have approved Zimhi 5 mg/0.5 mL, a high-dose naloxone injection product, for use in the treatment of opioid overdose.
According to Genentech, atezolizumab is the first and only cancer immunotherapy approved for adjuvant treatment of NSCLC.

Study indicates COVID-19 vaccination is important for individual protection and for reducing transmission within families.

According to GenSight, GS030 combines AAV2-based gene therapy with optogenetics to treat RP.

According to Ocular Therapeutix, Dextenza is now the first FDA-approved, physician-administered intracanalicular insert capable of delivering a preservative-free drug for the treatment of ocular itching associated with allergic conjunctivitis with a single administration for up to 30 days.
The study also failed to meet its secondary efficacy endpoints and no new safety signals were observed.

Allostatic load is caused by a number of different stressors, including social isolation, poverty, and racism, many of which are commonly experienced by individuals belonging to racial and ethnic minorities, according to the investigators.
Samples for the test are isolated from nasopharyngeal swabs, anterior nasal swabs, and mid-turbinate swabs.

The investigators said that these cells, referred to as lymph node resident memory T cells, have been demonstrated to counteract the spread of melanoma in mice.

Seronegative patients with COVID-19 who required low-flow or no supplemental oxygen receiving Ronapreve had significant reductions in viral load within 7 days of treatment.
Trials show a consistent efficacy profile and a consistent safety profile for RBX2660 in reducing the recurrence of C. difficile infection.

According to a press release from Mirum, maralixibat is the first and only FDA-approved medication to treat ALGS, a rare liver disease that affects 2000 to 2500 children in the United States.