Adult patients with Clostrioides difficile infection who received hematopoietic stem cell transplant also benefitted from frontline medications fidaxomicin or oral vancomycin.

Adult patients with Clostrioides difficile infection who received hematopoietic stem cell transplant also benefitted from frontline medications fidaxomicin or oral vancomycin.

New data from an unreviewed, preprint research article indicate first-dose immunization efficacy of the Pfizer-BioNTech coronavirus 2019 vaccine improves by 51% 13-24 days post administration.

Sanofi has agreed to bottle and package at least 125 million doses of the COVID-19 vaccine BNT162b2 from Pfizer and BioNTech.

Atoltivimab, maftivimab, and odesivimab-ebgn (Inmazeb), a monoclonal antibody cocktail ffor the treatment of adult and pediatric patients with Ebola virus infection, becomes the first drug approved to treat Ebola virus.

There is no perfect moment to timestamp the accelerated, massive movement toward finding and distributing the first vaccine candidates for the coronavirus 2019 pandemic.

Regional and state access assistance, improved research and collaboration, and individualized options for patients are key factors in adherence to PrEP.

Pivotal phase 3 trial results show benefits for once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI; Trelegy Ellipta) in patients with uncontrolled asthma compared with standard FF/VI (Breo Ellipta) therapy.
Pivotal phase 3 trial results show benefits for once-daily fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI; Trelegy Ellipta) in patients with uncontrolled asthma compared with standard FF/VI (Breo Ellipta) therapy.

The matching of optimized technologies and novel approaches has provided investigators with a framework to assess and understand neurologic conditions.

The combination therapy was approved for twice-daily administration via the breath-actuated Pressair inhaler in patients with COPD.

Approximately 1.7 million insured US patients are burdened with moderate to severe plaque psoriasis.
Biologic dupilumab plus non-cosmetic topical moisturizer associated with significantly improved symptoms and health-related quality of life in particular patients with atopic dermatitis.

Tildrakizumab was approved by the FDA as a subcutaneous therapy for patients with moderate-to-severe psoriasis.
Analysis of care in a routine clinical practice setting show that 40-week treatment of biologic dupilumab (Dupixent, Sanofi-Regeneron) plus noncosmetic topical moisturizer (emollient) is associated with significantly improved symptoms and health-related quality of life (HRQoL) in particular patients with atopic dermatitis (AD).

From 1990 to 2016, the rate of deaths due to adverse effects of medical treatment decreased 21.4% in the United States.
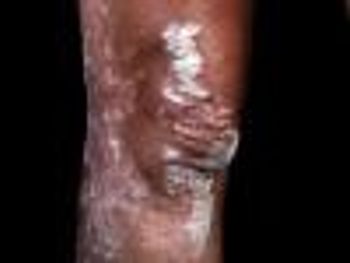
Patients administered guselkumab achieved an improvement of 90% or more Psoriasis Area Severity Index score than those administered secukinumab.

A recent study found further evidence that hepatitis C reinfection rates are greater in drug users despite successful treatment with direct-acting antiviral therapy.

New study finds that ocrelizumab (Ocrevus) has the capability to reduce risk of upper extremity disability progression in patients with primary progressive multiple sclerosis (PPMS).

The FDA expanded the label for eltrombopag (Promacta) to now include indication for adults and pediatric patients 2 years and older with severe aplastic anemia in combination with standard immunosuppressive therapy.

The US Food and Drug Administration (FDA) has approved the first generic medication indicated for lowering the risk of heart attack, or death from heart attack or stroke in patients with acute coronary syndrome (ACS).

The FDA has released a warning on cases of rare, serious genital area infections having been reported with sodium-glucose contransporter-2 (SGLT2) inhibitors.

FDA officials are requiring a warning about this on the prescription information.

Officials with the FDA have approved ivacaftor (KALYDECO) as the first and only therapy indicated to treat the underlying cause of cystic fibrosis in children aged 12 to

The designation was based on data from 7 clinical trials over the past decade, which assessed omalizumab’s efficacy and safety versus various food allergens, such as peanut, milk, and egg.

Midazolam Nasal Spray—from global biopharmaceutical company UCB, Brussels, Belgium (UCB)—has been previously granted Orphan Drug and Fast Track designations by the FDA

The FDA has granted approval to a pair of assays designated for the use with an immunodiagnostic system that detects hepatitis B (HBV) in individuals.

The FDA has approved the longest-lasting self-applied continuous glucose monitor (CGM) in the United States market.

The FDA has approved lusutrombopag (Mulpleta) for the treatment of thrombocytopenia in particular adults with chronic liver disease.

Each group reported a thrombosis-related adverse event rate of 1.9%.

Published: August 1st 2018 | Updated:

Published: August 1st 2018 | Updated:

Published: August 9th 2018 | Updated:

Published: August 13th 2018 | Updated:

Published: August 30th 2018 | Updated:

Published: March 2nd 2019 | Updated: