
The 4 academic centers will evaluate the role of telehealth in areas such as prevention, screening, diagnosis, treatment, and survivorship.
Jill Murphy is an associate editor for Pharmacy Times®. She graduated from Monmouth University in 2018 in communication and was previously a copywriter and communications specialist at a marketing agency prior to her start at the publication.

The 4 academic centers will evaluate the role of telehealth in areas such as prevention, screening, diagnosis, treatment, and survivorship.

Adalimumab-bwwd is a tumor necrosis factor blocker indicated for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn disease, ulcerative colitis, and plaque psoriasis.

Study finds that only 19.6% of US adults have high cardiovascular health, whereas 62.5% have moderate health and 17.9% have low.
Study shows that adult survivors of cancer had a 42% greater risk of CVD than people who did not have the disease.

Brexpiprazole is indicated as an adjunctive therapy to antidepressants in adults with major depressive disorder and as a treatment in adults with schizophrenia.
Many patients with heart failure are hesitant to receive the COVID-19 vaccine due to the fear of myocarditis.

Patients recorded their symptoms and use of acute medications in a headache diary.
Infection in pregnancy increases the risk for preterm delivery and other adverse pregnancy outcomes, including stillbirth.

The researchers noted that new therapies have faced challenges because of this knowledge gap in CHD.
The Sanofi-GSK vaccine demonstrated a favorable safety and tolerability profile throughout stage 1 and stage 2 of the VAT08 trial, according to the researchers.
The Opioid and Naloxone program at North Dakota State University School of Pharmacy aims to make a difference.

Social determinants of health (SDOH), which include social, economic, and physical conditions, have a major impact on people’s health, wellbeing, and quality of life.
Clinically meaningful results may be a huge step forward in the development of a new therapy for systemic lupus erythematosus.
Over a period of 10 years, substituting antioxidants lutein and zeaxanthin for beta-carotene was found to be more effective at lowering the risk of AMD progression.

There are more than 70 cases of monkeypox across 18 states in the United States, as of this week.

Pembrolizumab is an anti-PD-1 therapy that works by increasing the ability of the immune system to detect and fight tumor cells.
These results from 3 separate long-term pooled analyses of adult patients with ulcerative colitis (UC) and CD treated with ustekinumab (Stelara) were presented at Digestive Disease Week meeting in San Diego, California.

Renin-angiotensin-aldosterone system inhibitors found to reduce the risk of an aneurysm rupture by 18%.
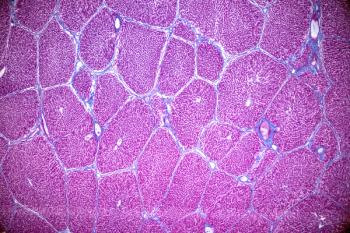
JZP458, a recombinant Erwinia-derived ASNase from a Pseudomonas fluorescens expression platform, was previously approved by the FDA for patients with ALL/LBL who had developed hypersensitivity to Escherichia coli (E. coli)–derived ASNase.

Additionally, the 2-time dependent variables, dose reduction, and relative dose intensity 2 (RDI2) were included in the respective model as covariates to explore the connection to overall survival.
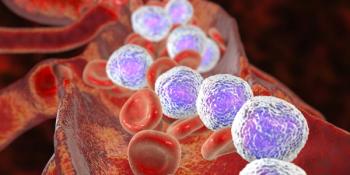
Overall, the investigators observed that the CR, undetectable minimal residual disease rates, progression free survival, and overall survival amomg the patients enrolled in the trial were favorable.
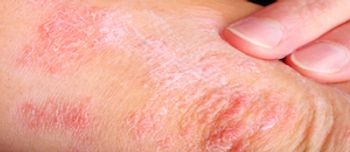
Further analyses showed guselkumab provided patients with sustained improvements in measures of health-related quality of life, fatigue, pain, and work productivity.

Biomarker positive patients were randomized to maintenance rucaparib 600 mg or matched placebo within 10 weeks of completing PBC until disease progression.
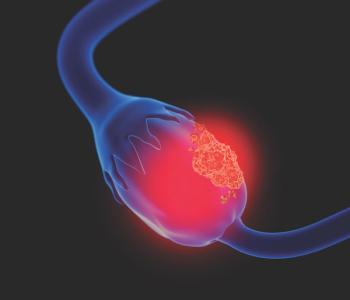
The researchers noted that the objective of the study was to assess the trends of 1L PARPi maintenance treatment uptake and PFS of patients with newly diagnosed AOC.

Individuals with elevated arterial stiffness combined with high blood pressure had the highest risk of developing type 2 diabetes.
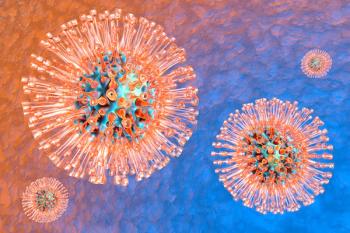
The new study analyzed KSHV’s latent-lytic switch, a process in which the virus exits its dormancy state to replicate in the host cell.
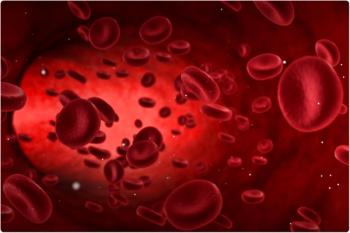
The safety profile of valoctocogene roxaparvovec in the phase 1/2 study was consistent with previously reported data, with no delayed-onset treatment-related adverse events (AEs).

These real-world findings help support the use of DPI in patients at increased risk of vascular events.
Efforts to decrease hospital readmissions may have been misguided because the United States are already doing better than other high-income nations in this area.
Any antibiotic use was associated with higher rates of IBD, and the risk went up substantially with each course.