Researchers identified an immune receptor that may lead to new drug treatments that target immune activation in patients with HIV.

Researchers identified an immune receptor that may lead to new drug treatments that target immune activation in patients with HIV.
A newly-developed biomedical device may help prevent chemotherapy from spreading away from the targeted tumor.

National Pharmacist Day, celebrated annually on January 12, honors pharmacists across different specialties and in every setting by recognizing the impact they have in health care.

Study shows the United States spends more on health care on a per capita basis than other developed countries, mostly due to higher prices.
High consumption of sugary beverages has previously been linked to obesity, diabetes, and some types of cancer.
A newly study published study aimed to distinguish between uninfected cells and latently infected cells, with hopes that eradicating the latent reservoirs could lead to a cure for the disease.

A survey of California 2017 enrollees estimates how drops in enrollment may affect premium increases.
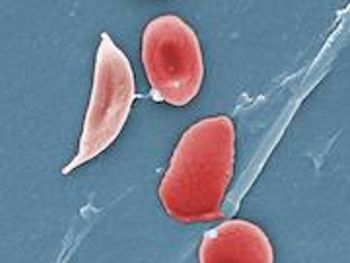
Officials with the FDA have granted Breakthrough Therapy designation to Novartis’ crizanlizumab (SEG101) for the prevention of vaso-occlusive crises (VOCs) in patients of all genotypes with sickle cell disease.
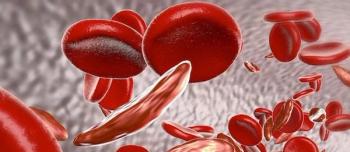
Officials with the FDA have granted Breakthrough Therapy designation to Novartis’ crizanlizumab (SEG101) for the prevention of vaso-occlusive crises (VOCs) in patients of all genotypes with sickle cell disease.
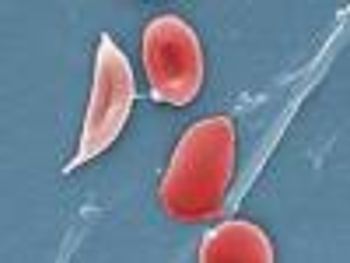
Crizanlizumab (SEG101, Novartis) is being developed to prevent painful complications called vaso-occlusive crises in patients with sickle cell disease.
Study suggests a new therapy could stop tumor growth by affecting myeloid cell behavior.

A multimethod approach including use of refill data may be the most effective method to identify nonadherence in young patients with chronic kidney disease.
Advancements in cancer therapy have improved survival time for patients with non-small cell lung cancer (NSCLC), according to a new study published in the Journal of Thoracic Oncology.
Controlling the viral reaction of cells latently infected with HIV may have implications for future drug treatments.
Advancements in targeted therapies have helped significantly improve survival in late-stage anaplastic lymphoma kinase positive non-small cell lung cancer.

Andexxa [coagulation factor Xa (recombinant), inactivated-zhzo] is the first and only antidote for patients treated with rivaroxaban or apixaban, when anticoagulation reversal is needed.

Andexxa [coagulation factor Xa (recombinant), inactivated-zhzo] is the first and only antidote for patients treated with rivaroxaban or apixaban, when anticoagulation reversal is needed.

Officials with the FDA approved Portola Pharmaceuticals’ Prior Approval Supplement (PAS) for the Generation 2 manufacturing process for Andexxa [coagulation factor Xa (recombinant), inactivated-zhzo].

Study finds at least a 1.5-fold difference between states with lowest and highest proportions of cancers related to obesity.
Researchers aimed to better understand the different ways that breast cancer avoids immune system detection.

Cigna recently announced the completion of its $54-billion purchase of Express Scripts.
Ravulizumab (Ultomiris, Alexion) is the first long-acting complement inhibitor approved for the treatment of paroxysmal nocturnal hemoglobinura.

Ravulizumab (Ultomiris, Alexion) is the first long-acting complement inhibitor approved for the treatment of paroxysmal nocturnal hemoglobinura.

Cigna recently announced the completion of its $54-billion acquisition of Express Scripts.

Cigna recently announced the completion of its $54-billion purchase of Express Scripts.

Officials with the FDA have approved olaparib (Lynparza, AstraZeneca and MSD) for the maintenance treatment of adult patients with BRCA-mutated advanced ovarian cancer.

Review highlights global patterns in excess body weight, drivers of the epidemic, and contribution to cancer burden.

With this approval, olaparib (Lynparza, AstraZeneca and MSD) is the first approved poly ADP ribose polymerase inhibitor for ovarian cancer.
Durvalumab plus tremelimumab did not meet the primary endpoint of the phase 3 MYSTIC trial evaluating improvement in overall survival and progression-free survival compared with chemotherapy.

Prucalopride (Motegrity, Shire) is the only serotonin-4 receptor agonist approved for adults with Chronic Idiopathic Constipation.