
The FDA has approved a cannabidiol (CBD) treatment for 2 rare and severe forms of epilepsy, making it the first FDA-approved medication that contains a purified drug substance derived from marijuana.

The FDA has approved a cannabidiol (CBD) treatment for 2 rare and severe forms of epilepsy, making it the first FDA-approved medication that contains a purified drug substance derived from marijuana.

Officials with the FDA have approved cannabidiol oral solution (Epidiolex) for the treatment of seizures associated with 2 rare and severe forms of epilepsy, Lennox-Gastaut syndrome, and Dravet syndrome, in patients aged 2 years and older. This is the first FDA-approved drug that contains a purified drug substance derived from marijuana, and is the first FDA approval of a drug for the treatment of patients with Dravet syndrome.

Epidiolex is the first FDA-approved drug that contains an active ingredient derived from marijuana and is the first approved treatment for patients with Dravet syndrome.

The findings highlight the role of Epstein-Barr virus (EBV) infection in multiple sclerosis pathogenesis and support therapies targeting EBV-infected immune cells.
Nivolumab plus low-dose ipilimumab demonstrated positive initial results in part 1 of a phase 3 study.

Researchers identified an epigenetic mechanism connected to a known genetic risk factor for multiple sclerosis.
Tonsillectomy was associated with an almost tripled relative risk for upper respiratory diseases, including asthma, influenza, pneumonia, and chronic obstructive pulmonary disorder.

Researchers investigated whether high body mass index is a risk factor not only for a greater risk of multiple sclerosis, but also for advancing to the secondary progressive stage of the disease.
A form of artificial intelligence known as a deep learning convolutional neural network outperformed dermatologists in diagnosing malignant melanomas.
Researchers identified a promising biomarker for the PARP-inhibitor sensitivity in small cell lung cancer, which may help in determining the appropriate treatment for select patients.
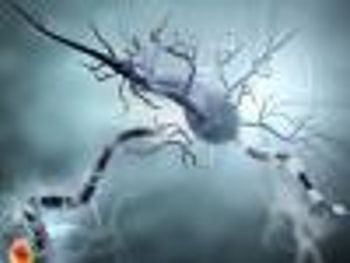
Researchers identified a primary mechanism of inflammatory demyelination in the central nervous system during multiple sclerosis, which may lead to new therapeutic approaches.

Researchers aimed to assess the relationship between fatigue in multiple sclerosis and inflammatory or other immunomediated markers.

In response to the Trump Administration’s drug pricing blueprint, the American College of Rheumatology issued a set of policy principles on drug prices and access to rheumatology treatment.

Despite universal access to care afforded to US military service members, there is a need to improve and expand access to PrEP for those patients at highest risk for HIV infection.

Officials with the FDA have granted accelerated approval to pembrolizumab (Keytruda, Merck) for 2 new indications, including the treatment of refractory primary mediastinal large B-cell lymphoma (PMBCL), and recurrent metastatic cervical cancer in previously treated patients whose tumors express PD-L1.

Pembrolizumab (Keytruda, Merck) gained FDA approval for 2 new oncology indications.
The Working Group examined the insulin supply chain and the factors that impact the costs of and access to insulin products.

Cemiplimab induced a response in approximately half of patients with advanced cutaneous squamous-cell carcinoma.

Researchers studied the effects of an antioxidant-enriched multivitamin on inflammation and health outcomes in patients with cystic fibrosis.
According to a new study, reducing prescription opioid duration, not dosage, may be more effective in curbing misuse after surgery.

The researchers found that total duration of opioid use was the strongest predictor of misuse, with each refill and additional week of opioid use associated with an increased rate of misuse.

Patients with both private and public insurers face high denial rates across the United States for life-saving hepatitis C drugs, despite changes in restrictions and increased price competition.

Indazole chloride may be able to reduce disability burden in patients with multiple sclerosis by producing good inflammation that promotes axon myelination.


Although precision medicine holds promise for advanced cancer care, many oncologists indicated challenges in the adoption of new, targeted therapies.
Drug shortages aren’t going away anytime soon, which is why it’s important for pharmacies to put preparedness plans in place ahead of future IV shortages, according to an interactive discussion held this week at the ASHP Summer Meeting and Exhibition.

For health care providers, staying up to date on drug development trends is crucial to understanding the implications of these therapies and how they can best help their patients.

Ibrutinib plus obintuzumab improved progression-free survival in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma.
The American Cancer Society (ACS) has updated its colorectal cancer (CRC) screening guidelines to lower the age at which individuals are recommended to begin screening.