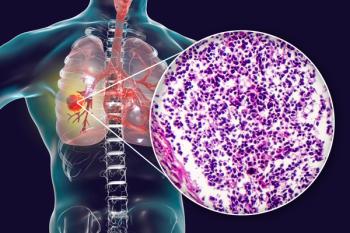
Neladalkib enters phase 3 trials, targeting advanced ALK-positive NSCLC, promising improved outcomes for TKI-naive patients with brain metastases.

Gillian McGovern is an editor at Pharmacy Times®. She graduated from Rowan University in 2023 with a BA in Writing Arts and concentrations in Publishing & Writing for the Public, Technical & Professional Writing, and Creative Writing.

Neladalkib enters phase 3 trials, targeting advanced ALK-positive NSCLC, promising improved outcomes for TKI-naive patients with brain metastases.
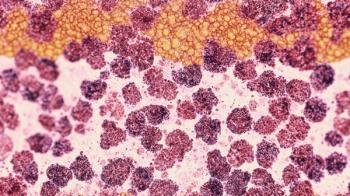
The CRL states that the STARGLO data do not provide sufficient evidence to support the second-line indication in the US patient population.

The biosimilar showed similar efficacy, pharmacokinetics, pharmacodynamics, safety, quality of life, and immunogenicity in patients with chronic spontaneous urticaria (CSU).
Patients with chronic kidney disease (CKD) were more likely to get vaccinated for influenza and pneumococcal following a health care provider’s recommendation.

The expanded instructions for use labeling expand on the Farawave and Farawave Nav Pulsed Field Ablation (PFA) catheters.

BCPPs can address behavioral health workforce shortages and advocate for expanded access and reimbursement amid evolving legislation and treatment landscapes.
This designation is significant because there are no FDA-approved treatments for transplant recipients with or preventing antibody-mediated rejection (AMR).

These health care professionals are crucial to optimizing safety in medication therapy, addressing policy barriers, and the collaboration with others in the field.
The long-term outcomes from the CARTITUDE-1 trial in multiple myeloma and durable complete response data from the STARGLO trial in diffuse large B-cell lymphoma can be significant for these disease states.
Steroids hindered the shrinkage of tumors in patients with non-small cell lung cancer (NSCLC) receiving immune checkpoint inhibitor (ICI) therapy.
The study found biosimilar initiation rose to nearly 42% from 2016 to 2023.

Sebetralstat becomes the first FDA-approved oral treatment for hereditary angioedema (HAE), offering rapid relief for patients experiencing acute attacks.
Patients with chronic kidney disease (CKD) receiving efepoetin alfa demonstrated a response rate of about 75.6%.

The data emphasize the idea that there are no safe exposure limits as well as the need for guidelines.

Real-world data show that patients switching from Humira to biosimilars Hadlima and Hyrimoz achieve similar outcomes without hospitalization.
The letter cited deficiencies previously identified at a third-party manufacturing vendor unrelated to oxylanthanum carbonate (OLC).
Pharmacists can help oversee the treatment process to ensure patients with transthyretin amyloid cardiomyopathy (ATTR-CM) are adhering to tafamidis.
Amyvid is used for brain imaging to estimate amyloid plaque density in patients with cognitive impairment undergoing evaluation for Alzheimer disease.

Health care professionals are encouraged to educate patients on potential cannabis-related risks to cardiovascular health.

Compared with prior intravenous formulations, the autoinjector is a more accessible administration method for children and their caregivers.

Hulio shows comparable efficacy and safety to Humira in treating chronic plaque psoriasis, supporting its FDA interchangeability status.
Lorundrostat reduces blood pressure and urine albumin-to-creatinine ratio in patients with chronic kidney disease (CKD) and hypertension.

Its approval was based on data from a 3-year trial that enrolled patients with alkaptonuria.
The authors note that the combinatorial principal component analysis (cPCA) may be effective in other complex diseases outside of chronic kidney disease (CKD).
The subcutaneous infusion is indicated for pediatric patients aged 6 to 17 years who weigh less than 60 kg.

Pharmacists and pharmacy technicians are key to offering more accessible and efficient care.

Ribociclib in combination with a nonsteroidal aromatase inhibitor (NSAI) improved invasive disease-free survival (iDFS) while allowing for better quality of life in hormone receptor-positive (HR+)/human epidermal growth receptor 2-negative (HER2−) early breast cancer.
Patients with chronic kidney disease (CKD) and type 2 diabetes receiving both treatments had greater reductions in urinary albumin-to-creatinine ratio.

Biologics and biosimilars have a major role in modern medicine because of their clinical benefits and economic impact on patient care.

Innovative strategies are essential to recruit pharmacy students and transform practices, ensuring equitable access to pharmacy services in underserved areas.