
Eladocagene exuparvovec-tneq is the first FDA-approved gene therapy for individuals with aromatic L-amino acid decarboxylase deficiency.
Ashley Gallagher is an editor at Pharmacy Times®. She graduated from St. Bonaventure University in 2020 in journalism and mass communications. Previously, she worked as a pharmacy technician for a retail chain.

Eladocagene exuparvovec-tneq is the first FDA-approved gene therapy for individuals with aromatic L-amino acid decarboxylase deficiency.
In the analysis, 34.7% of commercially insured patients with chronic obstructive pulmonary disease or asthma received an influenza vaccination.

Despite their proven abilities, organizations such as the American Medical Association still oppose so-called “scope creep.”
The designation follows 2 previous orphan drug designations for pancreatic cancer and soft tissue sarcoma.
Kristi Veis discusses the importance of flu vaccinations and the role of pharmacists in administering them.
Ocrelizumab (Ocrevus; Genentech) is used for the management and treatment of multiple sclerosis.
There were differences in the variables that influenced vaccination decision making for adults aged 18 to 26 years and for parents making the decision to vaccinate their child
Kristi Veis discusses the importance of flu vaccinations and the role of pharmacists in administering them.

Pegfilgrastim (Neulasta; Amgen) treats neutropenia that is caused by cancer medications and helps the bone marrow to create new white blood cells.
The study investigated faricimab-svoa (Vabysmo) as treatment of diabetic macular edema for patients in racial and ethnic groups that are often underrepresented in trials.
The most commonly reported errors included administering the wrong vaccine or administering an expired vaccine.

ASHP investigators determined the severity and impact of ongoing drug shortages, with approximately 99% of respondents reporting that they experienced a shortage.
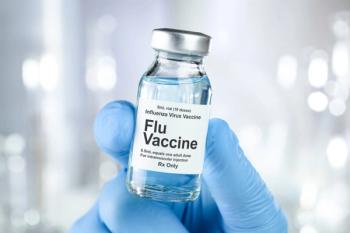
The simultaneous doses of both vaccines compared with individual RZV and quadrivalent high-dose inactivated influenza vaccine (HD-IIV4, Fluzone; Sanofi) had similar overall safety findings.

HLX14 (Organon) is an investigational biosimilar to denosumab (Prolia/Xgeva; Amgen).

Oral semaglutide had a safe and well-tolerated profile for individuals with type 2 diabetes, which is in line with those previously reported for the drug in other trials.

The new indications include pediatric individuals with acute lymphoblastic leukemia and polyarticular juvenile idiopathic arthritis.
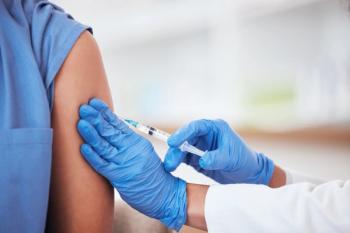
Champions for Vaccine Education, Equity, and Progress reported that the majority of pharmacists believe that it is important to prioritize vaccine development and distribution.

Investigators found that electronic letter reminders were most effective for older individuals, those who were unvaccinated in the preceding season, those with cardiovascular disease, and those with diabetes.
Glucagon-like peptide-1 (GLP-1) receptor agonists are widely known as treatments for type 2 diabetes, though some have been approved for indications beyond diabetes.
Orlynvah is approved for uncomplicated urinary tract infections caused by Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis.

The call follows the FDA’s sudden removal of tirzepatide from the drug shortage list, preventing compounding pharmacies from providing the drug.
The CDC Advisory Committee on Immunization Practice voted to expand the recommendation, which includes Prevnar 20 and Capvaxive.

The agency does not intend to take action against the plaintiffs for the violation of the Federal Food, Drug, and Cosmetic Act, including the compounding of tirzepatide.

Benefits include reducing serious health consequences, such as hospitalization and death, as well as the economic burden on health systems due to influenza infection.
The FDA previously accepted the biologic license application for CT-P13 as a subcutaneous formulation.

In April 2024, the FDA approved ustekinumab-aekn as a subcutaneous injection for the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis.
The additions include Dextrose 70% intravenous (IV) solution, Lactated Ringers IV Solution, and Peritoneal Dialysis Solution, which were affected by Hurricane Helene.

The gold standard of testing for food allergies is an oral food challenge test where patients consume the allergen, such as peanut extract, under supervision.

Investigators state that health care providers should consider the potential use of 2 doses to increase the effectiveness against influenza B.
The lawsuit alleges that the action was taken without the required notice and disputes the agency’s warning of “localized supply disruption,” while calling for more transparency.