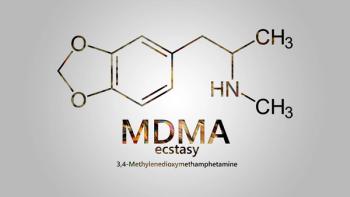
Ismail Lourido Ali, the director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), addresses the difference between legalization and decriminalization of psychedelic drugs, specifically in reference to the impact on medical research.