SkinTE could aid Wagner grade 1 diabetic foot ulcers by regenerating and activating the tissues surrounding the wound to heal it completely.

SkinTE could aid Wagner grade 1 diabetic foot ulcers by regenerating and activating the tissues surrounding the wound to heal it completely.

This marks the first FDA-approved treatment for this rare lipid storage disease.

Plasma therapy can involve burdensome procedures and infusions for patients, but pharmacists can use their expertise to make the process easier and more convenient.

Axatilimab is a colony-stimulating factor-1 receptor–blocking monoclonal antibody indicated after 2 lines of systemic therapy.

In children aged 1 to 3 years, the VP250 peanut patch was superior to placebo in the desensitization to peanuts and increasing the peanut dose that triggers allergic symptoms.

Using chromosome genomic array testing in combination with existing risk stratification models can better determine patients with myelofibrosis who can benefit from transplant.

The new data demonstrates the effectiveness of Mim8 in children with hemophilia A regardless of inhibitor status, expanding the populations in which the novel drug in development could have a treatment benefit.

67Cu-SAR-bisPSMA is indicated for adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC).
Treatment with semaglutide yielded superior effects on drinking outcomes compared with existing therapies to reduce alcohol cravings.

Using central obesity measures provides more accurate estimates of the proportion of CRC cases attributable to excess weight compared to using BMI alone.

There was an association observed between fluctuating low-density lipoprotein cholesterol and the risk of developing dementia and cognitive impairment.
Relapsed treatment has evolved with CAR T, bispecific antibodies, and immunotherapies.

Improvements in baseline for markers of hemolysis (indirect bilirubin, lactate dehydrogenase, and haptoglobin) were observed only in the mitapivat arm.

Semaglutide has been approved for reducing kidney disease progression and cardiovascular risk in patients with type 2 diabetes, marking a significant advancement in CKD management.
Priority review status will allow for expedited development of zongertinib, putting it on the path toward approval as the first in a new class of drugs for mutated NSCLC.
The 75 minutes of infusion time typically required is costly and difficult for patients.
T-DXd has a boxed warning for interstitial lung disease and pneumonitis.

PfSPZ-LARC2 is a genetically engineered malaria vaccine that marks a significant step forward in combating malaria around the world.
Educating patients about lifestyle modifications is a critical part of preventing cerebrovascular events.
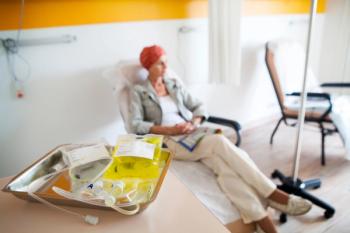
Using new treatment advances, traditional medications, genetic profiles, immunotherapies, and individualized plans enables providers to improve outcomes for patients with TPBC.
The test offers an accurate, fast, low-cost, and noninvasive tool for early diagnosis of pancreatic ductal adenocarcinoma.
Pharmacists play a vital role in supporting patients with endometriosis through guidance on treatment options and managing side effects.

Embedding a pharmacist in the dermatology clinic improved clinical and financial outcomes.

These findings may lead to new therapies to help reduce the risk of heart disease in women who are at risk.

Treatment selection includes patient factors, cost, adverse effects, and drug interactions.

QPL use in the pharmacy setting may enhance patient engagement while allowing pharmacists to showcase their medication-related expertise.
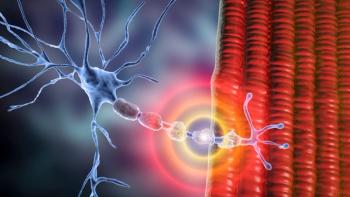
Identifying and managing IVIG-related side effects early on in treatment can improve efficacy and safety for patients with CIDP.

Coinfection with RSV and hRV is linked to a higher prevalence of lower respiratory tract infections, according to new data.
Many patients may struggle to understand the complex immunization schedules compiled by the CDC.