In trials, blood pressure reductions were maintained over extended periods, suggesting that the drug could offer a sustained effect.

In trials, blood pressure reductions were maintained over extended periods, suggesting that the drug could offer a sustained effect.
These findings are significant in understanding interactions between glucagon-like peptide-1 medications and thyroid dysfunction because prior research has shown conflicting results.

Methemoglobinemia is a blood disorder that affects proper release of oxygen around the body.

However, pharmacists can help mitigate these effects.

Trends across small cell lung cancer (SCLC) and diffuse large B-cell lymphoma (DLBCL) emphasize the need to further improve global treatment options.
The proposed rule would increase access to semaglutide for millions of Americans.

Because these cells are present throughout the body, there may also be implications for other cancer types.

The approval is based on clinical studies that demonstrate the management of heart rate with minimal reductions to blood pressure.
Lisocabtagene maraleucel chimeric antigen receptor T-cell therapy significantly enhances treatment of relapsed/refractory B-cell malignancies, offering high response rates and durable remissions.

Pharmacists are well-positioned to address diabetes from a multifaceted approach.
Once relatively obscure, PBMs are now front and center in debates over drug pricing and access. Their future role will have profound implications not only for how medications are priced but also for how pharmacies conduct their operations.
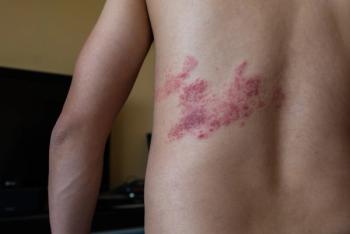
Although shingles is more common in adults, children who have had chickenpox or the chickenpox vaccine can develop shingles.

Augmentation is a condition in which RLS symptoms intensify or change in response to long-term dopamine agonist treatment.

Postpartum depression (PPD) rates also had notable increases in those with class I and class II/III obesity.

However, the monthly expenditure was not significantly associated with the financial incentives.
Investigators also found that vaccination for those aged 27 to 45 produces a higher number needed to vaccinate.

Pharmacists play a vital role in multidisciplinary health care teams by enhancing medication safety, optimizing therapeutic outcomes, expanding medication access, reducing costs, and improving patient education.

Exposure to pollutants PM2.5 and NO2 was found to increase the likelihood of developing a peanut allergy and of the allergy persisting.

At least 1 patient had a fraudulent tax return filed using illegally obtained personal information.

Oral phenylephrine is a common ingredient in OTC products, but its clinical efficacy is questionable. The FDA is considering removing it from the market.

This is the first oral liquid form of imatinib (Imkeldi; Shorla Oncology) to be approved to treat cancers.

Acoramidis is a novel, highly potent transthyretin stabilizer.
Findings highlight the unmet medical need for outpatient interventions and preventive measures that can reduce hospitalizations.

Recent studies suggest limited benefit post-myocardial infarction for patients with preserved heart function, prompting potential updates to treatment guidelines and reconsideration of long-term use.

Raymond Lorenz, PharmD, BCPP, discusses the role of pharmacists in bridging laboratory results and clinical practice, highlighting ongoing efforts to establish pharmacogenomic testing standards and foster multidisciplinary collaboration for broader clinical impact.

Jai N. Patel, PharmD, BCOP, CPP, discussed efforts to standardize the evaluation of clinical utility in pharmacogenetics, emphasizing its variability, the role of pharmacogenomics in research, and the importance of robust study designs for pharmacy professionals.
The disappearance could impact updates for surveillance strategies and vaccine development.

Community health care workers help support both primary care and school settings by assisting with medication administration and communications with staff.

An ACCP speaker explains that the FDA faces a multitude of challenges.

Expert discussed benefits of precision oncology treatments that utilize thousands of biomarkers to design personalized treatment plans.