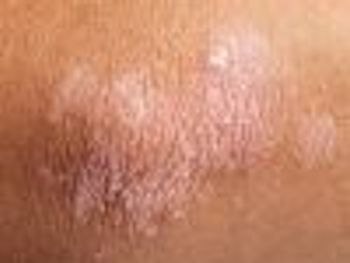
Treatment with secukinumab led to clearer skin after 12 weeks compared with placebo.

Treatment with secukinumab led to clearer skin after 12 weeks compared with placebo.
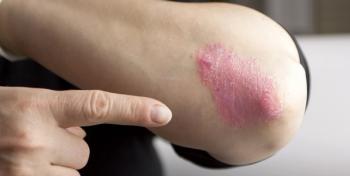
The FDA today approved Novartis' secukinumab (Cosentyx), an interleukin-17A antagonist, for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
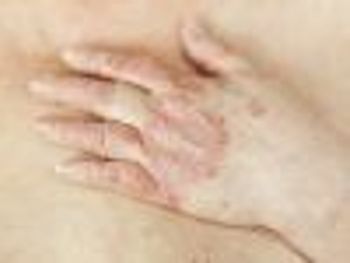


In a head-to-head phase 3b study, Novartis' secukinumab (Cosentyx) beat Janssen Pharmaceuticals' ustekinumab (Stelara) in clearing the skin of patients with psoriasis.
Patients with autoimmune conditions have an increased risk of developing shingles due to immune-suppressing treatment regimens.
More severe psoriasis associated with clinically significant cardiovascular risk.

Two doses of brodalumab generated better results in moderate-to-severe plaque psoriasis compared with ustekinumab (Stelara).

A long-term study of Celgene Corporation's apremilast showed that the oral selective inhibitor of phosphodiesterase 4 provides relief to adult patients with active psoriatic arthritis, as well as adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.

AstraZeneca and Amgen tracked psoriasis patients taking brodalumab, ustekinumab (Stelara), or placebo, and the results showed brodalumab produced the greatest results in clearing skin.
Methotrexate injection treats patients with rheumatoid arthritis, polyarticular-course juvenile idiopathic arthritis, and psoriasis.

The FDA's Dermatologic and Ophthalmic Drugs Advisory Committee has recommended the approval of Novartis' first-in-class secukinumab therapy for moderate-to-severe plaque psoriasis.
Patients with moderate-to-severe psoriasis are more likely to have uncontrolled hypertension.


Early diagnosis is the key to successful management of psoriatic arthritis, and treatment of this inflammatory disease is largely dependent on which symptoms are predominant.
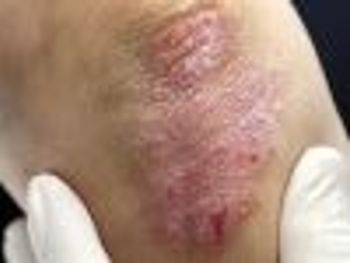
Patients who responded to brodalumab showed improvement in skin condition and joint swelling.
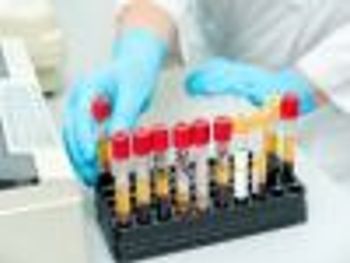
Recommendations for co-morbidities identified as an important step for the optimal care of patients.
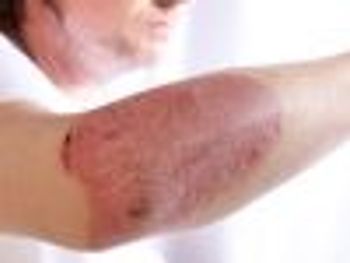
Methotrexate, a traditional disease-modifying antirheumatic drug, may enhance the likelihood of therapeutic persistence with novel biologic agents.

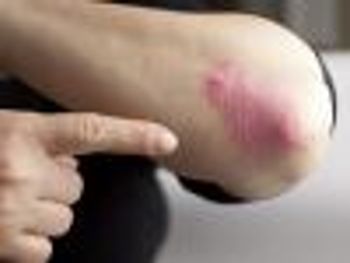
Questionnaires given to patients with psoriasis predicted probable psoriatic arthritis in a significant number of patients.
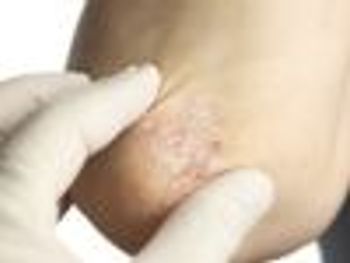
Biologic agent golimumab effective over 5-year span in treating disease symptoms and improving quality of life.
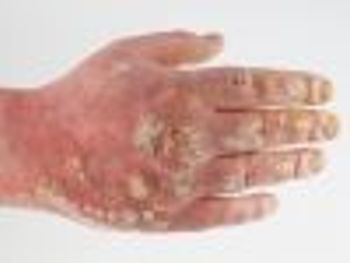
Patients with psoriasis, psoriatic arthritis, or both conditions felt that their quality of life decreased if they experienced condition symptoms in more parts of their body.


Left untreated, psoriasis can lead to serious medical complications.