
Top news of the day from across the health care landscape.

Top news of the day from across the health care landscape.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more.

Study finds that glecaprevir/pibrentasvir pan-genotypic efficacy for hepatitis C virus is achieved regardless of whether patients are concurrently receiving gastric acid-reducing drugs.

Trastuzumab and hyaluronidase-oysk (Herceptin Hylecta, Genentech) is indicated for subcutaneous administration in certain patients with HER2-positive early and metastatic breast cancer.

Approved by the FDA in July 2018, Perseris is the first once-monthly, subcutaneous risperidone-containing, long-acting injectable available in the United States.

Top news of the day from across the health care landscape.

The new class of smart drugs has the ability to bring the toxic payload directly to the cancer cells.

Continued consumption of peanuts was associated with positive quality of life and perceptions of safety following immunotherapy treatment for peanut allergy.
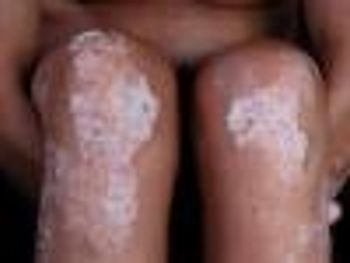
The controlled injection offers patients with plaque psoriasis the ease of self-injection.
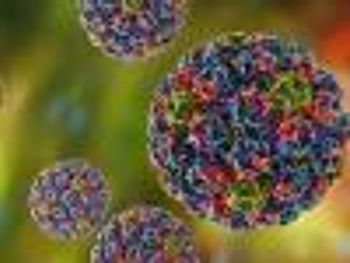
The findings reinforce the effectiveness of the HPV vaccine in preventing cervical cancer.

In a real-world setting, long-term outcomes were more favorable following early intensive therapy when compared with first-line moderate-efficacy diseased modifying therapy.
A 10 mg twice daily dose of tofacitinib (Xeljanz, Xeljanz XR, Pfizer) used in patients with rheumatoid arthritis increased the risk of blood clots in the lungs and death in a safety clinical trial.
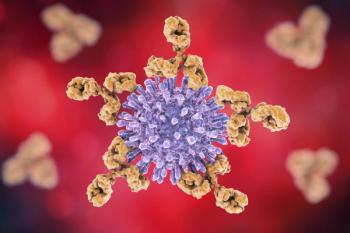
In order for a substantial decrease of HIV infections in the United States, there needs to be a rapid expansion of prevention and treatment for individuals at risk.

The trial aimed to evaluate the survival rate of patients with metastatic hormone-sensitive prostate cancer who were treated with the combination of enzalutamide and androgen deprivation therapy.

A recent study identified genetic changes in e-cigarette users similar to those seen in cigarette smokers.

Top news of the day from across the health care landscape.

This study demonstrates the significance and efficacy of breast cancer screenings for prevention and early intervention.

The FDA approved trifluridine/tipiracil (Lonsurf, Taiho Oncology), also known as TAS-102, as a treatment for certain adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma.
The combination of Opdivo and low-dose Yervoy was compared with sunitinib for a 30-month follow-up.

Study adds to the ongoing research on immunotherapy and its potential as a viable treatment for patients with cancer.
New research supports the use of direct-acting antiviral therapy for all individuals with chronic hepatitis C infection.

Ron Lanton III, Esq., executive director and senior counsel for Frier Levitt Government Affairs, explains the most effective way to facilitate DIR fee reform on a state and federal level.

Study explores the efficiency and accuracy of aspirin in colorectal cancer prevention.

Study highlights the importance of managing depression and anxiety to mitigate the effect on the cognitive function of patients with multiple sclerosis.

Top news of the week from Specialty Pharmacy Times.

The T cell marker could help lead the improved treatment for HIV and cancer patients.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

Polypharmacy can contribute to a higher risk of health problems, such as drug interactions and adverse effects.

Translational study of breast cancer therapy sought to ‘take research from bench to bedside.’