Tips for pharmacy professionals on how to best counsel patients who have concerns regarding their brain health.

Tips for pharmacy professionals on how to best counsel patients who have concerns regarding their brain health.

Rich Tremonte, the executive vice president and president of Community & Specialty Pharmacy at AmerisourceBergen, discusses the critical role community pharmacies are playing in helping to expand testing and reduce infections amid re-openings in the country.

Today, we’re celebrating Larisa Janoczkin, PharmD, a pharmacist with CVS is Howell, New Jersey.
The study results show improvements at each stage in the HPV vaccination series.

According to a new study by West Health and Gallup, 88% of US adults support the federal government directly negotiating the price of a COVID-19 treatment with a drug manufacturer upon its availability due to concerns regarding raised drug prices.

In the United States, 3.5 million people have been diagnosed with schizophrenia and it is a leading cause of disability.
Guselkumab is a monoclonal antibody that selectively binds to the p19 subunit of IL-23 and inhibits its interaction with the IL-23.

Student pharmacists across the country have had to adjust their expectations of what P4 year will look like.
Using artificial intelligence (AI) and automated monitoring, researchers and physicians at Oregon Health & Science University (OHSU) have designed a method to help people with type 1 diabetes better manage their glucose levels.

Research shows that the over-prescribing of opioids for short term pain management is a widespread issue in the United States.

Antibodies isolatied from plasma donated from recovered COVID-19 patients fights the virus that causes COVID-19.

Pharmacy managers will need to employ effective decision-making that will include evaluating vendors for post-purchase support, and discern the right time to purchase technology.
Giant Food has announced that all vaccinations recommended by the ACIP are once again available at its 153 in-store pharmacies across Virginia, Maryland, Delaware, and the District of Columbia.

individuals with four or more depressive symptoms had a 20% increase in cardiovascular events and death.

Galcanezumab-gnlm (Emgality, Lilly) has received FDA-approval for the preventive treatment of migraine in adults and for the treatment of episodic cluster headache in adults.

The participants were categorized according to their vitamin K levels and heart disease. However, those with the lowest vitamin K levels had a 19% higher risk of death, compared with those whose vitamin K levels reflected adequate vitamin K intake.

This is an important area for pharmacists to apply their knowledge of medications.
In addition to adhering to the CDC recommendations, pharmacies should consider workflow changes intended to minimize risk to both patients and the pharmacy staff providing immunization services.
The TEDDY study also aims to identify environmental triggers that cause the immune destruction of beta cells.

The expanded delivery services aim to allow customers to continue shopping while staying safely at home during the COVID-19 pandemic.

Gardasil 9 approved for the prevention of oropharyngeal cancer and other head and neck cancers caused by human papillomavirus types 16, 18, 31, 33, 45, 52, and 58.

Current treatment guidelines do not recommend the use of either drug in hospitalized patients with COVID-19 outside of clinical trials.

Dolutegravir tablets are intended to treat pediatric patients at least 4 weeks old and at least 6 pounds who have never been treated for HIV or who have been treated, but not with an integrase strand transferase inhibitor class drug, according to the press release.
Although flu vaccines have been proven to reduce severe illness, evidence is lacking on the link between flu vaccination and antibiotic prescribing at the population level in the United States, according to the study authors.

Which natural product or herbal medication could cause a severe phototoxic reaction to laser treatment?

The results of the real-world, observational study were presented by Eli Lilly during the American Diabetes Association's 80th Virtual Scientific Sessions.

Managing diabetes in rural areas is difficult due to limited access to specialty care and self-management programs.
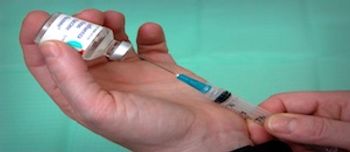
The CDC’s update to the Immunization Schedule highlights the new best practices and the efficacy and safety of crucial vaccines for children, according to a session at the virtual National Association of Pediatric Nurse Practitioners 2020 Annual Meeting.

This Tip of the Week examines a case where a pharmacist actually sued his state’s Board of Pharmacy.

Kimberly Erlich, MSN, RN, MPH, CPNP, PMHS, Nurse Practitioner, Pacific Coast Psychiatric Associates & the Healthy Teen Project, discusses pharmacogenomics and behavioral health in treatment of children and teens.