
The Pfizer COVID-19 vaccine booster dose is the same strength as the primary series of the Pfizer COVID-19 vaccine, with findings demonstrating the same level of safety as the primary series.

The Pfizer COVID-19 vaccine booster dose is the same strength as the primary series of the Pfizer COVID-19 vaccine, with findings demonstrating the same level of safety as the primary series.

The smartphone group were nearly 4 times more likely to have their wound infection diagnosed within 7 days of their surgery compared to the routine care group.
Individuals with diabetes and atrial fibrillation were less likely to notice symptoms of irregular heartbeat.

The difference in COVID-19 infection time between patients receiving heparin and those who did not was approximately 4 days on average.

Those with attention-deficit/hyperactivity disorder are much more likely to have GAD at some point in their lives than others who do not have it.
RSV causes an estimated 14,000 deaths and 177,000 hospitalizations annually among US adults over 65 years of age.
Breast cancer found to be the most expensive type of the disease, costing approximately $3.4 billion in the United States.
Bacteria, fungi, and other germs that cause endocarditis may enter the bloodstream through improper dental care, catheters, and IV drug use.

The Build Back Better bill include an authorization for the government to negotiate lower drug prices.

Social determinants of health can play a significant role in the treatment trajectory and outcomes for patients with cancer because oncology care requires close communication between the patient and clinicians.

Study finds COVID-19 booster shot was extremely beneficial for all patients with cancer, especially those with a blood cancer.
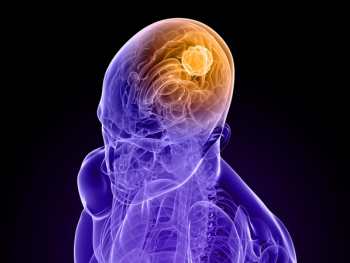
Approximately 40% of patients with stage IV melanoma have brain metastases at diagnosis.

Their statement also calls upon the business community to support the new federal requirement that individuals working for companies with more than 100 employees be vaccinated.
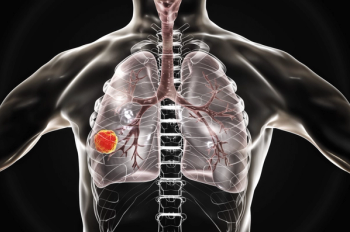
The results of a study showed that non-Hispanic Black patients treated with immunotherapy had a 15% lower risk of death from non–small cell lung cancer than non-Hispanic white patients.
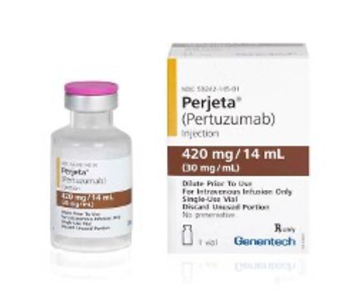
Pertuzumab is indicated for use in combination with trastuzumab and docetaxel for treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
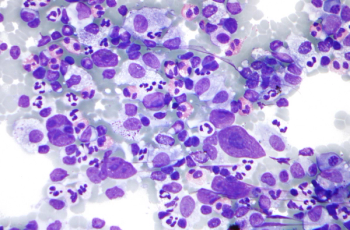
Hodgkin lymphoma is most common in people between 20 and 40 years of age and those over 55 years of age.

Pharmacy Times spoke to Michael Young, MBA, Vice President of Pharmacy Strategy, Parata Systems, about centralization and automation in the pharmacy field and how these tools can create new solutions for pharmacists.
Solutions could save federal government more than $1 billion annually for these single-source drugs.

Pembrolizumab demonstrated a statistically significant improvement in disease-free survival, reducing the risk of disease recurrence or death by 32% compared to placebo.

Molnupiravir (Lagevrio) is a potent ribonucleoside analog that blocks SARS-CoV-2 replication by acting as a competitive substrate of virally-encoded RNA-dependent RNA polymerase.
Treatment for endometrial cancer includes surgery, radiation therapy, chemotherapy, immunotherapy, and supportive care.

Pharmacists should keep an eye on the pipeline for autologous and allogeneic CAR T-cell therapies as well as antibody-specific treatments.

Facilities play key role in health care because of their bulk production, stringent quality standards and wide distribution.

Receipt of PrEP education or prescription relies heavily on a provider’s discretion, which can be subject to social biases.
Discontinued fentanyl infusion left attached to patient contributes to his death, but risk-reduction strategies can prevent similar mistakes.

Practicing mindfulness was associated with fewer psychological symptoms, such as posttraumatic stress, depression, and anxiety.
The panel also recommends considering risk factors for poor response, including uncontrolled disease, immunoparesis, the number of previous lines of therapy, and patient age.
In the IMPACT trial, women who were positive for high-risk HPV received a follow-up triage test to help determine whether their cervical cells were transforming to cervical pre-cancer.

The FDA initially approved regorafenib for chemorefractory metastatic colorectal cancer and unresectable gastrointestinal stromal tumor in 2012.

In addition to escalating workloads, the COVID-19 pandemic has exposed understaffing in the pharmacy profession.