
Article
Immunotherapy: Managing Immune-Related Toxicities in Patients
Author(s):
With the increasing use of these treatments in the oncology space, pharmacists should be knowledgeable about how to best manage patients on immunotherapy.
Immunotherapy has shifted the treatment paradigm for many different types of cancer. However, patients may experience related toxicities that are unique to these agents. With the increasing use of these treatments in the oncology space, pharmacists should be knowledgeable about how to best manage patients on immunotherapy.
A session held at the 2018 American Society of Health-System Pharmacists Clinical Meeting and Exhibition in Anaheim, California, gave an overview of the progression of immunotherapies and addressed appropriate treatment management strategies that pharmacists should consider.
To begin, Christine M Walko, PharmD, BCOP, FCCP, demonstrated the progression of immunotherapy over the years.
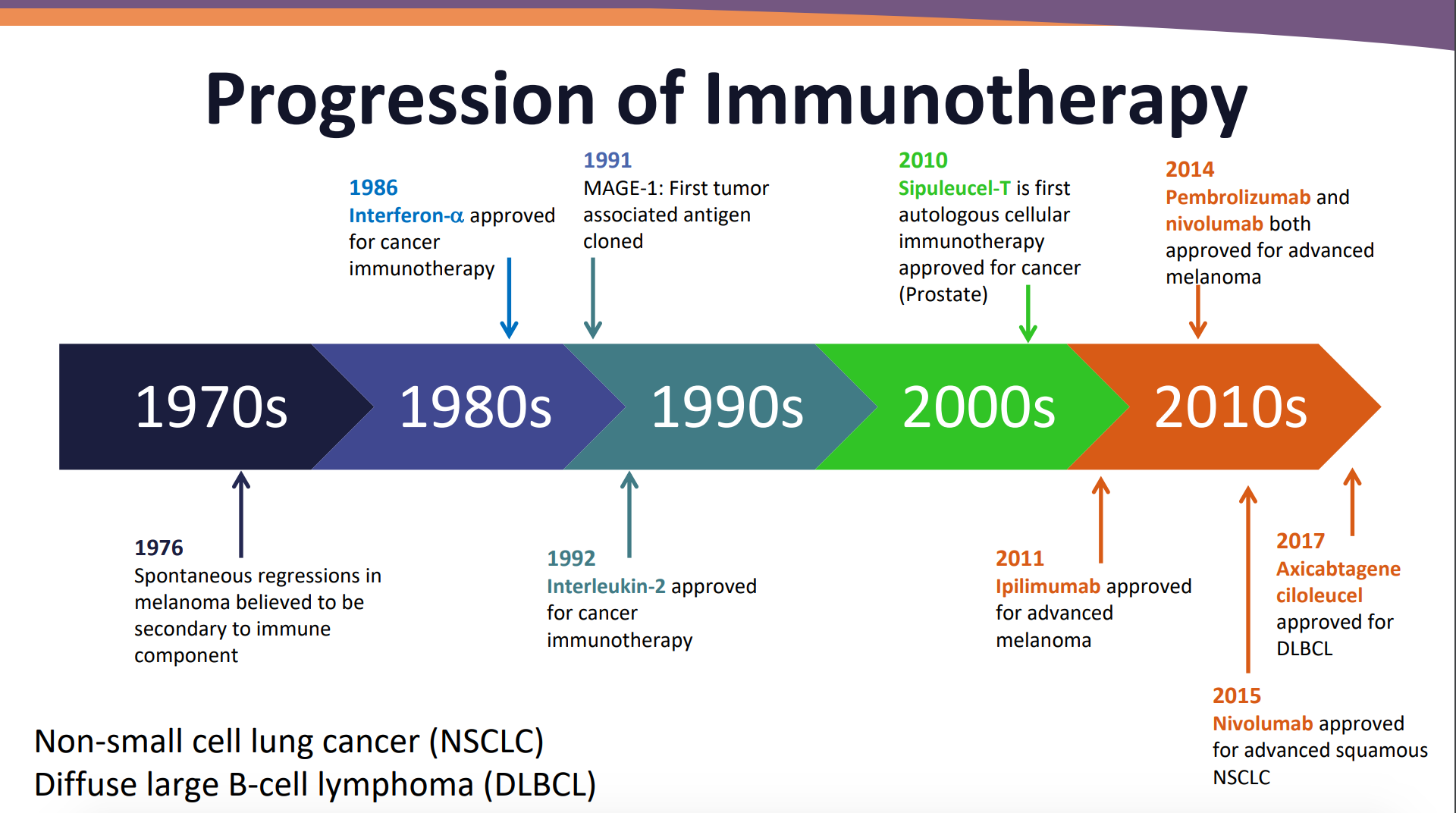
Credit: Walko CM, Kudchadkar RR. Evolving Role of Immunotherapy in Cancer Treatment. Presented at: Presented at: 2018 ASHP Midyear Clinical Meeting and Exhibition. December 2-6, 2018. Anaheim, California.
Advancements in the immunotherapy landscape have reduced the large incidence of toxicity that used to be seen. For example, approximately 85% of patients taking interleukin-2 therapies experienced grade 3 toxicities, compared with 26% in CTLA-4 therapy ipilmumab and 15% in PD-1/PD-L1 therapies nivolvumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
“We’re getting better, we’re getting more targeted,” Walko said.
However, toxicity exposure still remains a concern for patients receiving these therapies. Pharmacists are well-positioned to recognize the effects of toxicity and intervene with appropriate treatment strategies, according to the presentation.
In the presentation, Ragini R. Kudchakar, MD, provided an overview of some immune-related adverse events that may occur with immunotherapy.
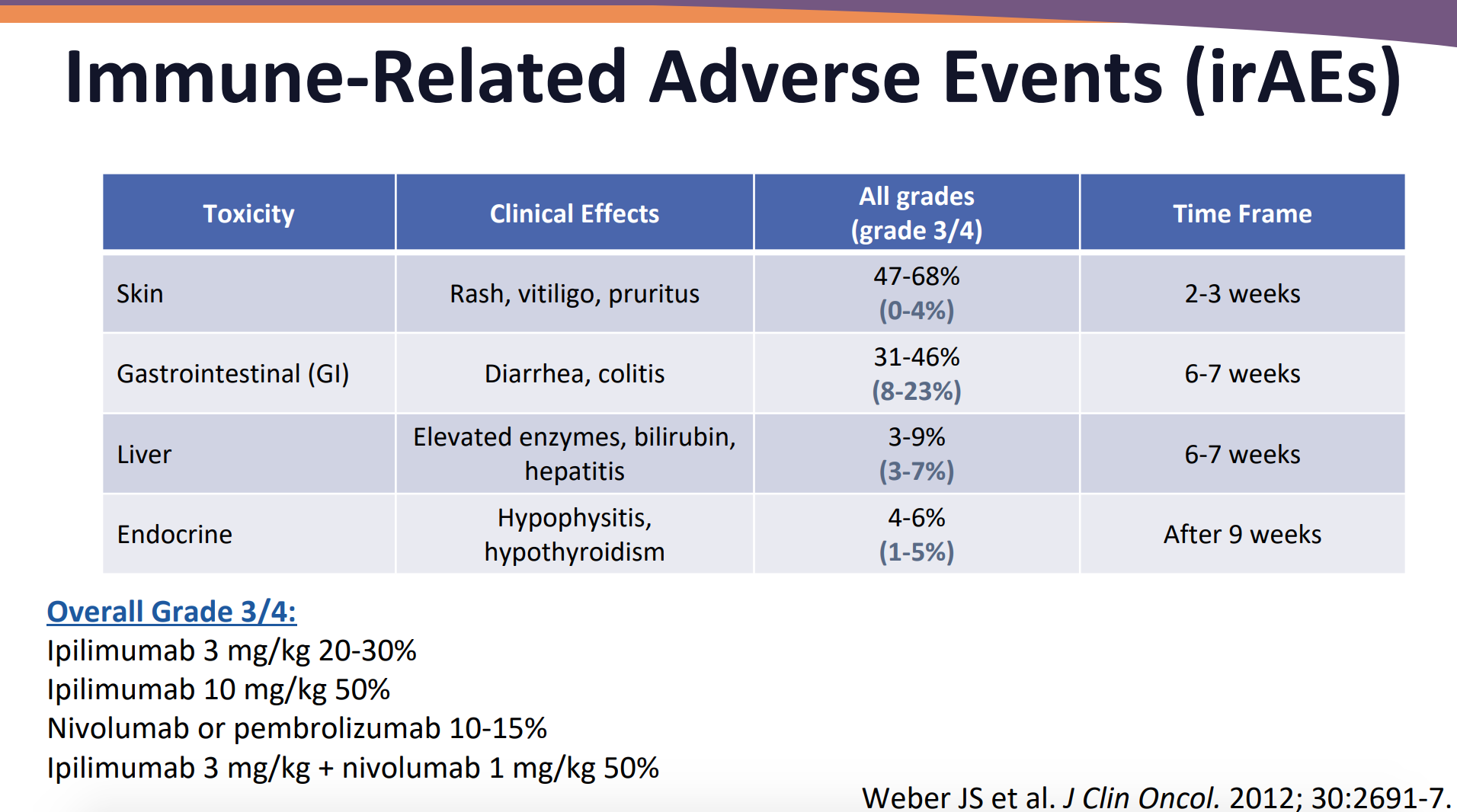
Credit: Walko CM, Kudchadkar RR. Evolving Role of Immunotherapy in Cancer Treatment. Presented at: Presented at: 2018 ASHP Midyear Clinical Meeting and Exhibition. December 2-6, 2018. Anaheim, California.
In addition, the American Society of Clinical Oncology guidelines offer recommended management strategies for immune-related adverse events occurring in patients treated with immune checkpoint inhibitors.
Some of the key guideline recommendations include:
- In general, checkpoint inhibitors can be continued with close monitoring for mild (grade 1) toxicities, with the exception of neurologic and some hematologic toxicities.
- For moderate (grade 2) toxicities, checkpoint inhibitors should be held until symptoms and/or lab values revert to grade 1 levels or lower. Corticosteroids may be offered.
- For severe (grade 3) toxicity, patients should receive high-dose corticosteroids for at least 6 weeks. Extreme caution when restarting immunotherapy after a grade 3 toxicity is recommended, if it is restarted at all.
- In general, very severe (grade 4) toxicity necessitates stopping checkpoint inhibitor therapy permanently.
- Consult the guidelines directly for more specific recommendations depending on which organ is affected.
Despite the increased risk of adverse events, data have shown that overall survival and time to treatment failure were not associated with immune-related adverse events or treatment with corticosteroids. Dr Kudchakar emphasized the importance of patient education and indicated that clinicians should maintain a high index of suspicion for immunotherapy as a cause for new adverse effects. Additionally, she noted that dose adjustments are not recommended after restarting therapy following toxicity.
Dr Walko explained that dosing remains a challenge for pharmacists, as the market is rapidly changing and dosing can vary between different approvals for a drug. For this reason, dosing information can change quickly.
“If you’re not used to working with immunotherapy all the time, double-check your dosing,” Dr Walko advised.
Given the high cost and toxicity of these agents, robust biomarkers will be essential in the future for optimizing therapy and selection sequencing, according to the presentation. A few biomarkers that have been looked at for response to immune checkpoint inhibitors include microsatellite instability, tumor mutation burden, and PD-L1 status. Other biomarkers currently being investigated include tumor infiltrating lymphocytes, DNA repair deficiencies and ARID1A mutations, and HLA class 1 genotype.
“I think the answer is there isn’t going to be 1 of these that’s going to be the best fit for everyone,” Dr Walko said.
PD-L1, for example, has correlated with outcomes but responses are still seen in PD-L1 negative patients. According to Dr Walko, future considerations should include standardized assays with consistent threshold values and differences between cancer types and PD-1 versus PD-L1 inhibitors.
She concluded that continued research into predictive biomarkers such as these will be key to balancing the clinical benefits and toxicities from immunotherapy in the future.
Reference
1. Walko CM, Kudchadkar RR. Evolving Role of Immunotherapy in Cancer Treatment. Presented at:
Presented at: 2018 ASHP Midyear Clinical Meeting and Exhibition. December 2-6, 2018. Anaheim, California.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.





