Gut microbiome research is no longer an obscure field in cancer care.

Gut microbiome research is no longer an obscure field in cancer care.

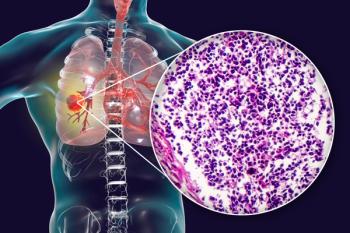
A patient with BPDCN finds her champion.
Advancements include novel combination therapies and improved diagnostic techniques.

Their expertise complements multidisciplinary teams in a variety of clinical settings.
The adjuvant therapy has demonstrated varied efficacy for patients.
These therapies are being investigated in earlier lines, with several new treatments in development.
An overview of current FDA-approved ROS1-targeted therapies.

Dispensing, communicating, and recording information may look different at each center.
The therapy offers new hope in solid tumor treatment with fewer adverse effects.

Peer Reviewed
Non–FDA-approved medications may be accessed for patient care via 3 alternative pathways: expanded access, the Right to Try Act, and off-label use, which are reviewed in this article.
Peer Reviewed
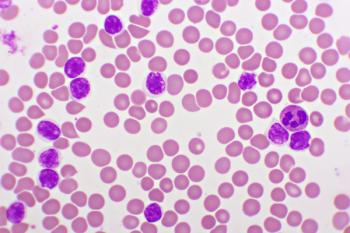
This review discusses the significance of the FDA-approved drug inotuzumab ozogamicin in pediatric patients with acute lymphocytic leukemia (ALL) and relapsed/refractory (R/R) ALL, and the evolving therapeutic options in R/R ALL.
Peer Reviewed
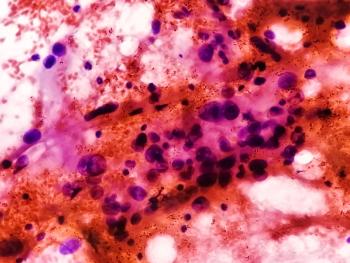
This retrospective cohort study provides preliminary evidence for safe removal of mesna from VAdriaC cycles, as the incidence of hemorrhagic cystitis did not increase in patients with Ewing sarcoma who received cyclophosphamide without prophylactic mesna.

Peer Reviewed
Artificial intelligence holds immense potential to address the rising demand for oncology services and improve patient outcomes by facilitating more effective, efficient, personalized cancer care.

The authors of these peer -reviewed papers collectively emphasize the evolving landscape of oncology treatment.