
Genome sequencing shows the clonality of outbreak isolates within each of 6 classes, which confirmed that recent transmissions accounted for high carriage.

Genome sequencing shows the clonality of outbreak isolates within each of 6 classes, which confirmed that recent transmissions accounted for high carriage.

The pharmacist's role in the profession has immensely changed since the COVID-19 pandemic, and it will continue to change with new initiatives for the future and the integration of more public health policies.
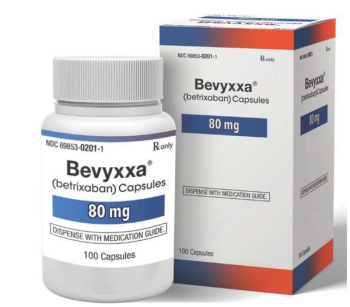
Betrixaban (Bevyxxa) is a factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism in adult patients.

Move Free is intended to support joint health and help with osteoarthritis pain.

On average, patients visit their community pharmacist 12 times more often than they do their primary care provider
Obesity and a limited physical activity are key factors in post-COVID-19 recovery.

Phase 3 REST-ON Trial data show that the medication demonstrated clinically meaningful improvement in assessments of disrupted nighttime sleep compared with the placebo.

Suzanne Soliman, PharmD, welcomed back guests Christina Madison, PharmD, FCCP, AAHIVP, and Joanna Lewis, PharmD.

Those hospitalized for COVID-19 were 21% more likely to develop shingles.
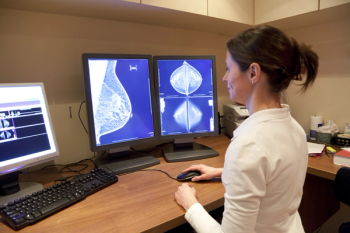
Study finds that health care providers need to reinforce the importance of annual mammograms among patients who survived breast cancer.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses some of the barriers patients with cancer may experience that oncology pharmacists can help them navigate through.

If all HHC agencies adopted policies requiring staff influenza vaccinations, the researchers predicted an 11.25% reduction in the rate of hospital transfers due to respiratory infections.

During this round, students were allowed to collaborate with their teammates to come up with the answers, and they wagered their points with each question.
Medication reconciliation and pharmacist counseling are crucial to clinical management of patients with hematologic malignancies.

Recent studies that indicated the efficacy of IVIG in patients with myelin oligodendrocyte glycoprotein antibody-associated disease used pediatric cohorts, with few evaluations of the treatment approach in adults.

Study captures the immune effect of the third dose of the COVID-19 vaccine in patients with plasma cell disorders and blood cancers.

Rabeprazole (AcipHex) can treat erosive or ulcerative gastroesophageal reflux disease, heartburn, stomach ulcers, and esophageal damage.

Coenzyme Q10 is an antioxidant the body produces naturally.
Pharmacists who educate patients with multiple sclerosis and expand their understanding and knowledge of the condition empowers them to take part in a more active role in managing their health.
Melissa Johnson, MD, program director of Lung Cancer Research at the Sarah Cannon Research Institute, discusses her presentation at the Community Oncology Alliance 2022 conference on new drugs in the non-small cell lung cancer space.

Jim Schwartz, RPh, executive director of pharmacy operations for Oncology Pharmacy Services, discusses his presentation at the Community Oncology Alliance 2022 conference on the new world of oral cancer drug dispensing.

BioXcel plans to launch Igalmi sublingual film in the United States in the second quarter of 2022.

Interested individuals may gain certification or continuing-education credits, many of which are available through the American College of Veterinary Pharmacists.

Additional support is necessary to ensure that infrastructure development continues and that all pharmacists have the necessary tools to continue to provide immunization services.

A recent meta-analysis of statins showed that prevalence of statin intolerance is less than 10%, according to the panel.
Drug meets primary endpoint at 50 mg with reduction in low-density lipoprotein levels from the baseline in the ETESIAN phase 2b trial

Valbenazine (Ingrezza) is indicated for the treatment of adults with tardive dyskinesia.

Aftersun Aloe Vera naturally soothes sunburns, minor skin irritations, and abrasions.
Regeneron’s supplemental biologics license application for the drug targets patients aged 12 years and older.

Alpelisib is the first FDA-approved treatment for PROS, which is a range of rare conditions characterized by overgrowths and blood vessel anomalies.