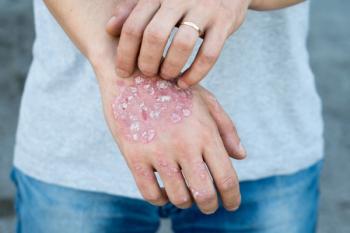
Due to the disease’s chronicity, the variability in treatment response and drug safety profiles, and the availability of multiple therapies, switching or discontinuing treatments is common in psoriasis.

Due to the disease’s chronicity, the variability in treatment response and drug safety profiles, and the availability of multiple therapies, switching or discontinuing treatments is common in psoriasis.
Comorbidities and cognitive impairment worsen non-adherence to anti-seizure medications in older adults.

The study will be conducted in Brazil, where PrEP use for HIV continues to be lower than desired.
The novel antibody-drug conjugate is the first to demonstrate overall survival benefits in patients who were already treated with therapy.

There are still significant unresolved issues in artificial intelligence and machine learning, which must be addressed before they can be safely and effectively used more broadly in medicine.
The 2022-2023 COVID-19 season did not have a significant surge of COVID-19 like it did early on in the COVID-19 pandemic.
Vaccination is especially important for older adults with any chronic health conditions because vaccines can prevent serious illness and resulting complications down the road.

Illegal online sellers threaten the health, privacy, and security of patients by failing to comply with the laws of the jurisdiction in which they are located, or the jurisdiction in which they are selling their medicines.

The study shed light on the importance of the source of vaccine information and other factors that prevent lifesaving vaccination.
Data from the expanded trial suggests that the NVX-CoV2373 COVID-19 vaccine protects against multiple viral variants.

Mental health interventions are a critical part of the global response to HIV, with multiple potential roles for psychiatrists and other mental health professionals.
It is possible that the pandemic had a positive impact on influenza vaccination rates.

At least 58% of mice treated with combined CRISPR therapies showed signs of complete HIV-1 viral elimination.
The therapy had a clinically meaningful response in patients with relapsed or refractory (R/R) follicular lymphoma and R/R mantle cell lymphoma.

Ivacaftor is now the first and only cystic fibrosis transmembrane conductance regulator modulator approved for this age group.

Clearer skin on the face and hands lasted 1 year into treatment.

PSG speakers present findings of the report at AXS23 and explain some of the notable results.
The infection causes diarrhea and inflammation of the colon, and it can be difficult to get rid of it.

Gregory also discussed the differences between specialty pharmacy distribution and physician’s office distribution, and the benefits of each for patients.

In addition to streamlining and strengthening patient care, Dave said working in a medically integrated pharmacy can be incredibly rewarding for pharmacists.

More than half of patients with Alzheimer disease administered donanemab showed a significantly reduced cognitive and functional decline.
Study examines association between breast cancer survival and neighborhood-level measures such as socioeconomic status.

Dr. Christina Madison speaks with Walter Oronsaye PharmD, RPh, known as the Phit Pharmacist, about nutrition and social media as a pharmacist.

Study sheds light on the importance of the source of vaccine information and other factors that prevent lifesaving influenza or pneumococcal vaccination.
Misinformation on YouTube promotes women wiping front to back as a non-evidence-based hygiene practice to prevent urinary tract infection.

The advent of pharmacy-based community health workers has helped to expand and codify the pre-existing community-based efforts of pharmacy technicians to reach even outside of medication access.
In addition to 2 FDA-approved options, several clinical trials are investigating other potential treatments for immunoglobulin A nephropathy.

Mendelian randomization makes it possible to infer if a drug target protein will have an on-target effect on disease-relevant traits.
Arexvy is the first respiratory syncytial virus (RSV) vaccine approved in the United States for the prevention of lower respiratory tract disease caused by RSV in individuals 60 years of age and older.

Asking pharmacists to do even more work while paring down their support is not a sustainable solution to increasing costs.