
Mama Village Rx & Wellness cares for Mamaroneck community, owner considers patients like family.

Mama Village Rx & Wellness cares for Mamaroneck community, owner considers patients like family.

Approximately 50% of patients with refractory or relapsed disease achieved complete response following treatment with the Janus kinase 1 (JAK1) inhibitor.

In 2018, brentuximab vedotin was approved for advanced Hodgkin lymphoma based on an improvement in progression-free survival of 82.3% in combination with chemotherapy.

A counterfeit medication was reportedly purchased at a retail pharmacy, appearing to have contained another type of diabetes medication that led to an adverse reaction.

Investigators found that individuals who received metformin were approximately 40% less likely to develop long COVID than those who received a placebo.

More than 4 times as many people who used the app quit smoking compared to a traditional support service.

Leqembi is an intravenous injection for the treatment of Alzheimer disease.

Standard prediction models that assess an individual’s risk of developing dementia and how to prevent it are limited in their ability to do so, showing high error rates and a higher chance of receiving a false-positive result, according to a recent study.

The FDA approved a molecular test for emergency use and a supplemental new drug application for a blood sugar medication.
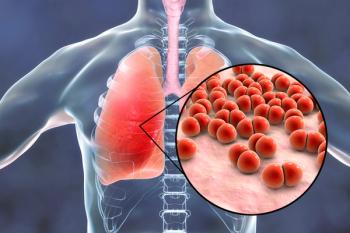
Immunocompromised status still appears to be one of the highest independent risk factors for pneumococcal pneumonia in middle-aged and older adults.
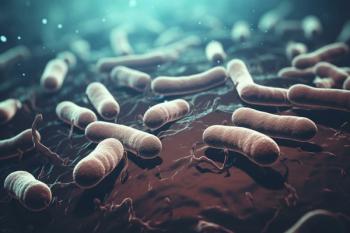
The novel antibiotic combines aztreonam, a monobactam β-lactam, with avibactam, a broad-spectrum β-lactamase inhibitor.

QR codes were invented almost 30 years ago and are used on everything from cereal boxes to repair manuals, yet pharmacy has yet to even consider them.

Multivitamins were associated with increased cellular oxygen consumption and more optimal blood nutrition biomarkers.
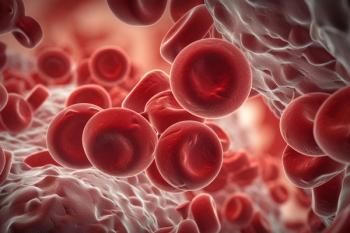
Trial results demonstrated that 58.5% of individuals receiving luspatercept-aamt (Reblozyl; Bristol Myers Squibb) achieved the primary endpoint of red blood cell transfusion independence of at least 12 weeks.

Current social determinants of health found to have a significant impact on sleep health.
How pharmacy teams can remove cost barriers to cardiac care.

Pharmacists can help patients live a life of meaning and gratitude after cancer recovery.
Best practices to safely, legally source prescription medications in Mexico.
Products treat ulcerative colitis, urinary tract infection, and psoriatic arthritis.

Liam Volk, MPharm, president-elect of the Pharmacists’ Defence Association LGBT+ Network, discussed how pharmacists can best support their LGBTQ+ colleagues and patients.

Family members should also be trained in how to administer seizure first aid, apply a tourniquet to control bleeding, operate an automated external defibrillator, and perform cardiopulmonary resuscitation.
The single institution study compared fixed dose capecitabine to standard dose capecitabine to compare efficacy and tolerability in metastatic breast cancer.
The benefit of ribociclib was seen as consistent against all of the stratification factors in the phase III study.
Heidi Finnes, PharmD, BCOP, FHOPA, addresses how the study data may impact the treatment of IDH1/2 mutating gliomas going forward.
Panelists discuss the potential impact of the study data presented at the ASCO 2023 Annual Meeting, which led to significant response from the field.

Andre Harvin, PharmD, MBA, discussed the use of immunotherapies in melanoma and broader challenges with access to oncology care in rural or underserved communities.
Ryan Haumschild, PharmD, MS, MBA, discussed his presentation at the Oncology Pharmacists Connect meeting, taking place June 15 through 17 in Austin, Texas.
The ASCO abstracts were on the TALAPRO-2 trial (abstract 5053) and PROpel trial (abstract 5012).

With immune checkpoint inhibitors in particular, patients are seeing greater results with fewer adverse effects.
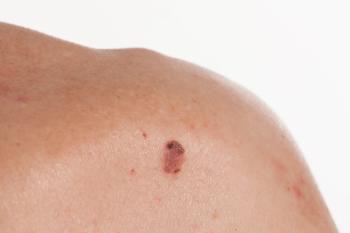
For squamous cell carcinoma systemic therapy that is not concurrent with radiation therapy, preferred regimens are immune checkpoint inhibitors.