
The authors emphasize that clinical research should focus on real-world difficulties, and pediatric asthma control should be seen as a health care priority.

The authors emphasize that clinical research should focus on real-world difficulties, and pediatric asthma control should be seen as a health care priority.
Safety and efficacy findings on Lomecel-B will be presented at the upcoming 2024 Alzheimer’s Association International Conference, which will be held July 28 to August 1.
Pharmacists have become the driving force behind immunizations in the US.

Acetylcholine deficit observed in patients with anorexia nervosa suggest potential targeted treatment options.

Proactive strategies to protect health care data from hackers.

Anatomical alterations result in many downstream changes, such as changes in pH, gastric volume, food intake, surface area for absorption, gastric emptying and transit times, and intestinal enzymes and efflux pumps, among others.

A large portion of the population of patients in the United States with GERD have the non-erosive type of the disease.

This month's product news features clonidine hydrochloride, ThermaCare HeatWraps, and iberogast.

The Pharmacy Quality Alliance Annual Meeting highlighted social determinants of health, oral oncolytics, and screening opportunities.

IMROZ study data show Isa-VRd may be a new standard of care.

Tune into this episode of “Public Health Matters” to hear how Walmart is leveraging its network to provide HIV care and specialty pharmacy services and how integrating community pharmacy outreach is significant for patients.

Investigators found that the risk of long COVID decreased over the course of the pandemic.
NADINA trial results support a new standard of care for these patients.
The investigators note that the high prevalence of long COVID, including in pregnant individuals, emphasizes a need for health care professionals and patients to closely monitor symptoms.
Radiotherapy may have applications beyond symptomatic relief when used in combination with immunotherapy.

Currently, limited data show the effect of adverse drug events in the outpatient setting.

This manuscript will describe the creation and development of a longitudinal sterile compounding rotation at an academic medical center for postgraduate year 1 acute care pharmacy residents.
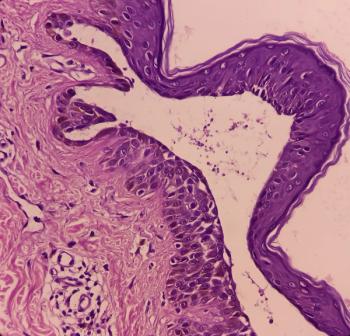
A patient with severe and recurrent pemphigus vulgaris achieved lasting remission with a novel intravenous immunoglobulin preparation that had the added benefit of reducing treatment-related side effects.


Investigators compared the criteria for eligibility in the VICTORIA trial with guideline and label criterion, finding a wider than expected population with heart failure were eligible for vericiguat therapy.

Leaders Must Continue to Prioritize Empathy and Human Connection.

Use of semaglutide injections may be linked to an increased risk of blindness in patients with diabetes.

Allison Burns discusses how pharmacists should prepare and educate themselves regarding OTC naloxone, and the steps that can be taken in stores and pharmacies to ensure OTC naloxone is visible and available.

In a meta-analysis, high-dose eicosatetraenoic acid and docosahexaenoic acid had the highest efficacy and lowest dropout rate among pharmacological interventions for migraine.

On a scale from 1 to 10, respondents rated their feelings of burnout an average of 7.25 in the second annual Pharmacy Times Burnout and Mental Health Survey.

Prediction model can accurately predict the risk of nerve damage in patients who received taxane-based chemotherapy.

Accurate medication reconciliations are critical for ensuring safe patient outcomes, and a correct medication list can help prevent drug-drug interactions, contraindications, and duplicate therapies.

Pharmacists and Pharmacy Advocates Have Been Calling for Pharmacist Practitioner Scope of Practice and Reimbursement for Decades, but What Happens When We Catch the Bus?


Data presented show significant advancements in treatment options.