Skin Cancer
Latest News
Latest Videos

Podcasts
CME Content
More News
Nivolumab plus relatlimab does not significantly improve recurrence-free survival in patients with stage 3, 4 melanoma.
A new study reveals the combination therapy enhances safety and efficacy for high-risk BRAF-mutated stage II melanoma.
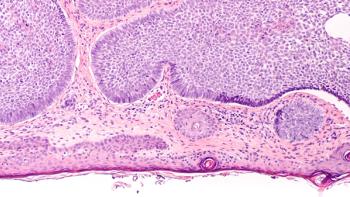
Pretrained foundation machine learning models significantly improved the accuracy of diagnosing non-melanoma skin cancer from digital pathology images and may offer a practical, resource-efficient solution for cancer diagnosis in underserved settings.
Christine Barrett, PharmD, BCOP, addressed advances in the treatment of stage III melanoma that provide the potential to improve long-term outcomes by enhancing immune response, tailoring surgical approaches, and refining patient selection strategies.
The FDA’s accelerated approval of lifileucel (Amtagvi; Iovance Biotherapeutics) marks a major milestone in immunotherapy for metastatic melanoma, building on decades of research in tumor-infiltrating lymphocyte therapy.
James P. Allison and the Discovery of CTLA-4: How Immune Checkpoint Blockade Revolutionized Oncology
Immunotherapy pioneer James P. Allison, PhD, revolutionized cancer treatment by discovering immune checkpoint blockade, leading to significant advances in survival rates and ongoing efforts to optimize immuno-oncology strategies.
Advancements in neoantigen vaccines and immunotherapy are transforming cancer treatment by leveraging genomic sequencing to overcome tumor heterogeneity and therapeutic resistance.
Building on a previously granted orphan drug designation, the latest FDA action for amezalpat puts the drug in a position for regulatory approval.
Vusolimogene oderparepvec in combination with nivolumab is being assessed to treat individuals with unresectable or metastatic stage IIIb-IV cutaneous melanoma.
New data shows that, in high-risk patients with advanced cutaneous squamous cell carcinoma, cemiplimab reduces the risk of disease recurrence and death.
Pharmacists play a vital role in cancer prevention and early detection by educating patients on risk factors, counseling on screening guidelines, and promoting adherence to evidence-based recommendations for common cancers, such as breast, cervical, colorectal, lung, prostate, and skin cancer.

The late former president played a critical role in advancing cancer care, as well as efforts to eradicate preventable diseases.
The new designation for the selective PPAR⍺ antagonist follows positive phase 1b/2 clinical trial results.
Calcipotriol in combination with 5-FU triggers Th2 immunity to prevent skin cancer.
The approval offers patients with solid tumors an alternative to intravenous PD-1 inhibitor administration.
Currently, the treatment is undergoing evaluation in a phase 2a trial.
Pembrolizumab is an anti-programmed death receptor 1 (PD-1) therapy designed to increase the immune system’s ability to detect and fight tumor cells.
Ten-year follow up data showed a sustained survival benefit with nivolumab-ipilimumab in advanced melanoma.
An ESMO 2024 presentation explores the growing role of the gut microbiome in immunotherapy response, emerging diagnostic tools such as PCR chips, and innovative therapies such as fecal microbiota transplantation and microbiome-preserving strategies to improve patient outcomes.
Subcutaneous administration of atezolizumab and hyaluronidase-tqjs had similar efficacy to intravenous administration.

OBX-115 could offer further treatment options for individuals with advanced or metastatic melanoma by enhancing persistence, antitumor activity, and clinical safety of TIL cell therapy.
Pharmacists play a vital role in therapy selection.
The trial is evaluating the success of BNT111 and cemiplimab in treating unresectable stage III or IV melanoma whose disease had progressed following anti-PD-(L)1-containing treatment.
Advancements in treatment include immune checkpoint inhibitors and BRAF/MEK inhibitors, though resistance remains a challenge, with lifileucel recently approved for previously treated metastatic cases.

NADINA trial results support a new standard of care for these patients.