C. difficile
Latest News

Video Series

Latest Videos
Podcasts
CME Content
More News
RBL is the first FDA-approved microbiota-based live biotherapeutic product for adults.
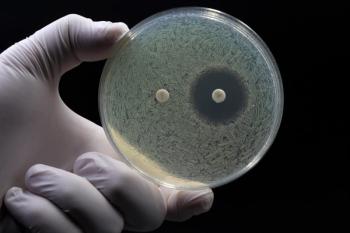
Educating the public about the dangers of self-medication and the importance of finishing prescribed antibiotic courses plays a crucial role
Pharmacists play a key role in the use of live biotherapeutic products, including fecal microbiota, live-jslm for prevention of recurrent Clostridioides difficile (C difficile) infection.
The BLING III trial investigated continuous vs intermittent β-lactam antibiotic infusions in critically ill patients with sepsis, finding no significant difference in 90-day mortality but suggesting potential benefits in clinical cure rates.
Scientists hypothesize that fecal microbiota transplant restores the recipient’s gut microbiota to an environment that mimics the healthy donor’s microbiota

Antimicrobial stewardship efforts focusing on accurate penicillin allergy histories, β-lactam cross-reactivity education, and guideline-driven narrow-spectrum prescribing may help sustainably decrease health care–associated Clostridioides difficile infection rates.

Living organisms rely on iron for survival and replication. Can new research discoveries disrupt C. difficile’s ability to maintain iron homeostasis?
Targeted use of ultraviolet light and sporicidal treatment can reduce disease transmission alongside existing interventions.
Sharon Rimon, NP, shares insights on the rectal administration of fecal microbiota therapy, highlighting its efficacy, ease of adoption in clinical practice, and benefits for patients.

Pharmacists can also ensure proper education and selection of therapy before and after discharge.
Since 2000, as the number of CDIs has surged, morbidity and mortality also have increased.
From newsworthy moments to groundbreaking research, these were the most-read Clostridioides difficile-related articles on Pharmacy Times in 2023.
Study Investigates Amiodarone As a Potential Toxin Inhibitor for Clostridioides difficile Infections
Amiodarone also provides pan-variant protection against toxins, a potentially growing area of research in C. difficile infection treatment.
In the study, a new policy empowered unit-based pharmacists to evaluate the patient’s needs and, when safe, switch from a PPI to an H2 blocker.
Feldman, who previously had C. diff, urges individuals to begin having conversations to help remove the stigma of the disease and raise awareness of its prevalence.

Feldman details her experience with C. diff, how the COVID-19 pandemic slowed the timeline of care, and how her surgical history and the use of antibiotics played into the disease’s onset.
The bacteria were shown to contain a membrane-bound organelle structure that is like those found in eukaryotic cells.
Iberzaplstat was non-inferior to standard of care vancomycin, was safe and tolerable, and will advance into a phase 3 trial.
Paul Feuerstadt, MD, FACG, AGAF, discusses insights in current treatment practices for CDI at the Peggy Lillis Foundation’s 2023 State of C diff Virtual Town Hall.

Christian John Lillis, co-founder and executive director at Peggy Lillis Foundation, discusses the role of the pharmacist in education that advances C diff awareness in the medical community.

Routine screenings for C diff on admission to the hospital could possibly prevent C diff transmission in the hospital setting.
Study Data Suggest That Cancer, Irritable Bowel Disease Are Associated With Risk of C Diff Infection
Patients who are immunocompromised generally had worse risk and severity of CDI.
With a new administration protocol created specifically for the routine clinic, infectious disease physicians can treat recurrent clostridioides difficile infection in less than 3 weeks.
Among individuals who received systemic antibiotics within 8 weeks, 6 months, 12 months, and 24 months of receiving fecal microbiota, RBX2660 prevented recurrence.