Eye Care
Latest News
FDA Approves Nufymco, Interchangeable Ranibizumab Biosimilar, for Retinal Diseases
Latest Videos

CME Content
More News

If approved, SYD-101 would have been the first FDA-approved treatment for pediatric myopia, offering hope to millions of affected children in the US.

Discover effective solutions for dry eyes, contact lens care, allergy relief, and conjunctivitis treatment in these insightful case studies.

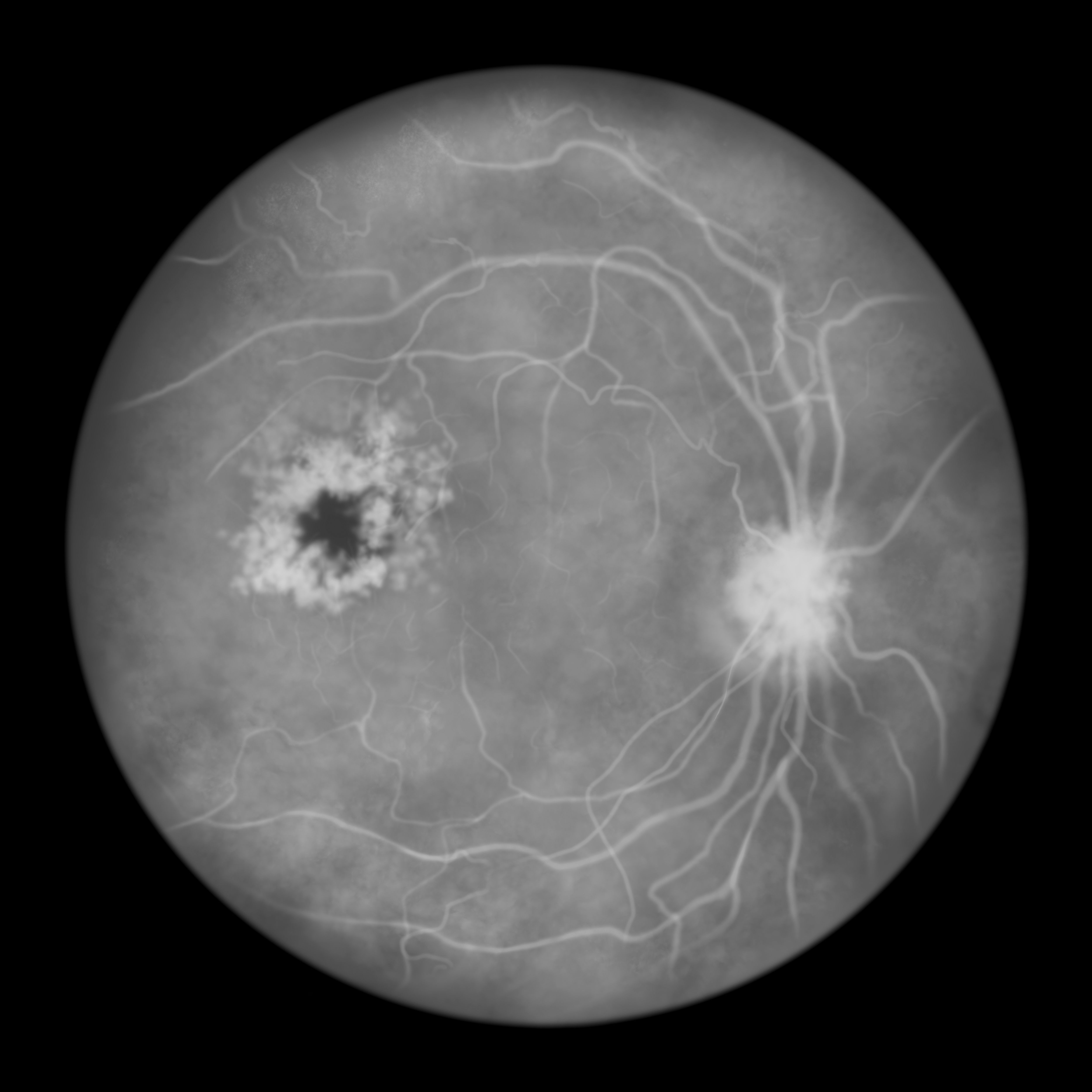
The mean time intervals between injection and complete retinal vascularization were similar between the biosimilar and its reference product.
The FDA approves Eydenzelt, a biosimilar for retinal diseases, enhancing treatment options for diabetic macular edema and age-related macular degeneration.

Demodex blepharitis is a common condition of eyelid inflammation. Lotilaner is the first FDA-approved, targeted treatment, offering effective mite eradication with minimal side effects.
Pavblu, a biosimilar to Eylea, also showed no clinically meaningful differences in efficacy, safety, and immunogenicity in patients with neovascular age-related macular degeneration (nAMD).
The recombinant shingles vaccine not only reduces herpes zoster and herpes zoster ophthalmicus but is also associated with lower risks of hospitalization for stroke and myocardial infarction in adults aged 50 and older.

The FDA approves aceclidine ophthalmic solution 1.44%, the first aceclidine eye drop for presbyopia, offering a new solution for millions struggling with near vision loss.
A recent study reveals Mvasi's comparable safety profile to Avastin for retinal diseases, highlighting the need for on-label biosimilars in ophthalmology.

Patients with diabetes using glucagon-like peptide-1 (GLP-1) drugs face double the risk of developing neovascular age-related macular degeneration, highlighting crucial ocular health concerns.

FDA approves acoltremon ophthalmic solution, a groundbreaking treatment for dry eye disease, enhancing natural tear production and patient quality of life.
Ranibizumab injection (Susvimo; Genentech) could offer long-lasting vision preservation and reduce the need for frequent injections in patients with diabetic retinopathy.
New research reveals that B vitamins and choline may slow glaucoma progression, offering hope for innovative treatments beyond traditional methods.

Cimerli offers a promising biosimilar treatment for age-related macular degeneration, enhancing vision preservation with its effective VEGF inhibition.
No manufacturing or safety issues were identified, but the FDA stated the new drug application did not demonstrate efficacy in adequate and well-controlled studies.
With the new designation, OpCT-001 can receive expedited investigations into its potential to improve vision function in patients with primary photoreceptor diseases.
The approval is based on results from 2 phase 3 clinical trials that showed revakinagene taroretcel’s efficacy in macular telangiectasia type 2 (MacTel).
The results act as a contradiction to conventional wisdom of high-density lipoprotein (HDL) cholesterol being healthy and lowering the risk of heart disease, but more research is warranted.

Avacincaptad pegol intravitreal solution is a prescription eye injection used to treat geographic atrophy.
NK is a degenerative corneal disease resulting from diminished corneal innervation.

The regenerative medicine advanced therapy designation expedites the development and review of regenerative medications that have the potential to address unmet needs for serious or life-threatening diseases.

Successfully integrating biosimilars into practices requires careful planning and execution, as they impact virtually all aspects of a practice.
Dyslipidemia is a condition in which there are abnormal levels of lipids in the blood stream, including high levels of low-density lipoprotein cholesterol, low levels of high-density lipoprotein cholesterol, and/or high levels of triglycerides.
The study investigated faricimab-svoa (Vabysmo) as treatment of diabetic macular edema for patients in racial and ethnic groups that are often underrepresented in trials.
Treatment with valacyclovir for a year decreased the risk of new or worsening eye disease by 26%.