Pharmacists will play a growing role in the use of biosimilars and guiding patients through the switching process.

Pharmacists will play a growing role in the use of biosimilars and guiding patients through the switching process.
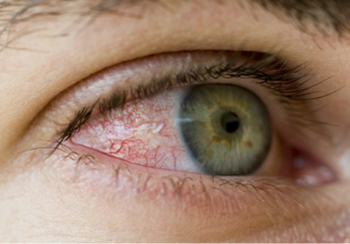
Eye drops come in many forms and for many indications, such as redness, itchiness, dryness, allergies, dilating drops, glaucoma drops, lubricants, numbing agents, antibiotics, and to lower pressure.

Aflibercept injection (8 mg) in a 12-week dosing regimen was found non inferior in vision gains compared to 8-week dosing regimen with 2 mg.

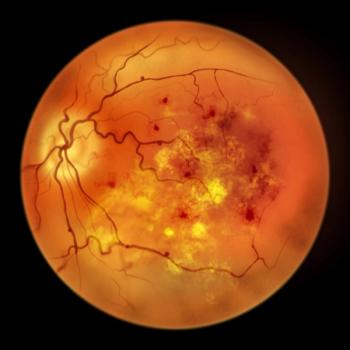
The approval marks the first and only treatment for geographic atrophy, a leading cause of blindness.

Aflibercept injection is also indicted for the treatment of neovascular age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema, and diabetic retinopathy.

Bausch + Lomb Corporation and Modulight anticipate that ML6710i will be available for eye care professionals during the first half of 2023.

Analysis focuses on why some individuals with a genetic predisposition develop AMD, while others are spared.
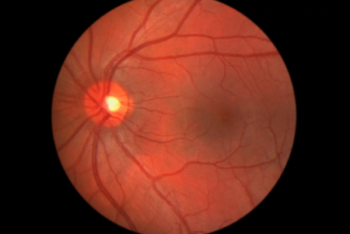
Retinal vein occlusion is the second most common cause of vision loss from retinal vascular diseases.

Aflibercept 8 mg demonstrated non-inferiority in vision gains in both the 12- and 16-week dosing regimens.

Iheezo is a sterile, single use ophthalmic gel preparation.

Omidenepag isopropyl ophthalmic solution (Omlonti) eye drops are indicated to reduce elevated intraocular pressure in patients with primary open-angle glaucoma or ocular hypertension.

Findings suggest potential screening and disease detection opportunities.
Treatment with faricimab-svoa (Vabysmo) may improve vision with fewer injections and treatment sessions for patients with wet age-related macular degeneration compared to other treatments.

The treatment gives patients with neovascular age-related macular degeneration a more affordable option, the companies say.
Over a period of 10 years, substituting antioxidants lutein and zeaxanthin for beta-carotene was found to be more effective at lowering the risk of AMD progression.

Genentech has released new 2-year data for individuals with diabetic macular edema and wet age-related macular degeneration.

Faricimab-svoa targets and inhibits 2 disease pathways that drive diabetic macular edema and neovascular or “wet” age-related macular degeneration.

According to the investigators, Susvimo is the first and only FDA-approved treatment for wet AMD that offers as few as 2 treatments per year.
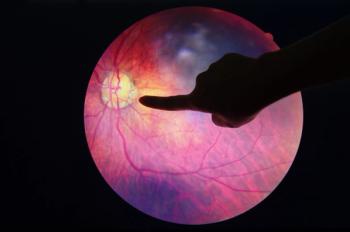
An anti-vascular endothelial growth factor therapy, ranibizumab prevents vision loss in patients with retinal vascular disorders, which can cause irreversible blindness or visual impairments in adults.

Levodopa may reduce the need for painful injections in patients with age-related macular degeneration.

By 2050, statisticians indicated that approximately 22 million people will be affected by macular degeneration or wet AMD.

Brolucizumab is the most clinically advanced humanized single-chain antibody fragment. This type of antibody is highly sought after due to their small size, enhanced tissue penetration, rapid clearance from systemic circulation, and drug delivery characteristics.

Estimates suggest that by 2020, 1.5 to 1.75 million people in the U.S. will be living with wet AMD, a leading cause of blindness worldwide and a rapidly growing public health concern, according to Novartis.

Those with hepatitis B virus have a significantly elevated risk for developing any type of age-related macular degeneration, according to a new report