
Did you know that health plans completely cover certain OTC products when patients present a prescription?

Did you know that health plans completely cover certain OTC products when patients present a prescription?

Warnings about the risk of ketoacidosis and serious urinary tract infections have been added to the labels of SGLT2 inhibitors.

FDA previously recommended Qualgen cease all sterile compounding until it corrected unsanitary conditions and poor sterile production practices.

Qualgen has taken corrective actions concerning its compounding facility conditions and sterility processes.

The FDA has once again delayed its enforcement of product tracing requirements for pharmacies.


October is American Pharmacists Month, the perfect time to promote the pharmacy profession and recognize the important role of the pharmacist.

Season 27 of "The Simpsons" opened with Homer separating from Marge and falling in love with his pharmacist.

Old drugs with new price hikes add another dimension to the national drug pricing debate.

The first biosimilar to receive FDA approval is now available the United States.

Pharmacists have many opportunities for collaborating with nurse practitioners and physician assistants on managing chronic and acute conditions commonly seen in the retail health practice setting.

If a projected 92% past participant return rate is any indication of an event's value, then success is once again in store for the third annual NACDS Total Store Expo.
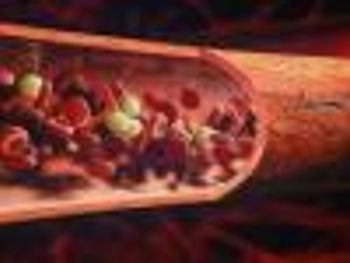
Promacta is the first and only oral thrombopoietin-receptor agonist that increases platelet production.

The FDA has approved Novartis's eltrombopag (Promacta) to treat a rare, chronic blood disorder in children.

Actavis's memantine hydrochloride extended-release and donepezil hydrochloride (Namzaric) treatment for moderate to severe Alzheimer's disease is now available for patients and health care professionals across the United States.

Actavis will immediately relaunch its budesonide inhalation suspension, a generic version of AstraZeneca's Pulmicort Respules, after a federal court upheld that a key patent is invalid.

Levoleucovorin is for rescue use after high-dose methotrexate therapy in bone cancer.
Drug treats rare but life-threatening condition.
Sandoz has introduced its ready-to-use levoleucovorin injection to the US market.
Isavuconazonium sulfate (Cresemba) is now available to treat 2 rare yet life-threatening fungal infections.

The drug is indicated for cardiac event prophylaxis or treatment in adult patients in the United States with heparin-induced thrombocytopenia.
Hospitals can now order Actavis's ceftazidime-avibactam (Avycaz) to treat serious infections in adults.

Novo Nordisk's liraglutide (Saxenda) is now available by prescription in the United States.
The JNC 8 guidelines are based on 27 large clinical trials that are often discussed in isolation, but have never been aggregated into a single source until now.

Four fast-acting liquid Mucinex medicines have been voluntarily recalled by RB due to mislabeling.

Experimental drug facilitates transfer of oxygen to cells and tissues.
The FDA has granted orphan drug designation to Prolong Pharmaceuticals' sickle cell disease treatment.

Almost all New York pharmacists are prepared to accept only electronic prescriptions for controlled substances, but 94% of prescribers are not prepared to issue them.

A 20 mg dose of vilazodone HCl (Viibryd) is now available in pharmacies to treat adult patients with major depressive disorder.

From optimizing inhaler technique in patients with chronic obstructive pulmonary disease, to catching red flags when dispensing controlled substances to patients with chronic pain, pharmacists can improve patient outcomes in an evolving treatment landscape.

Published: August 5th 2014 | Updated:

Published: August 5th 2014 | Updated:

Published: August 6th 2014 | Updated:

Published: August 8th 2014 | Updated:

Published: August 11th 2014 | Updated:

Published: August 11th 2014 | Updated: