
Approximately 74% of patients with chronic rhinosinusitis with nasal polyps receiving depemokimab across 2 trials did not need additional intervention.

Approximately 74% of patients with chronic rhinosinusitis with nasal polyps receiving depemokimab across 2 trials did not need additional intervention.
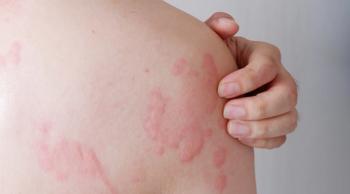
Although the 16-week end point was not met, tezepelumab demonstrated meaningful effects in patients with chronic spontaneous urticaria (CSU) after 32 weeks.
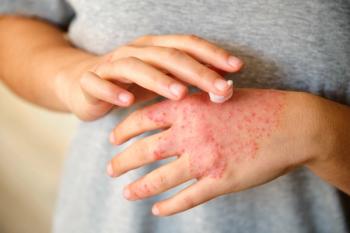
Efficacy and tolerability of topical treatments can vary by region, particularly sensitive areas such as the face and neck.

Tezepelumab demonstrated efficacy compared with placebo when treating patients with chronic rhinosinusitis with nasal polyps.

At week 8, significantly more children, adolescents, and adults who applied 1.5% ruxolitinib cream versus vehicle achieved IGA treatment success.

Patients with hereditary angioedema who received sebetralstat earlier had faster complete resolution of their attacks.

Detailed findings will be presented at the American College of Allergy, Asthma & Immunology (ACAAI) Annual Scientific Meeting.
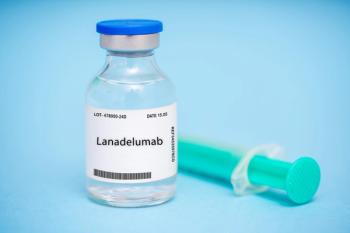
The data comes from 2 phase 4 trials, ENABLE and EMPOWER, which evaluated lanadelumab and the outcomes of treatment in adolescents with hereditary angioedema.