Once-Daily Oral Pill for Plaque Psoriasis Shows Promise
Deuvacracitinib (Sotyktu), which the FDA approved in September 2022, is a first-of-its-class tyrosine kinase 2 inhibitor and a member of the Janus kinase family.
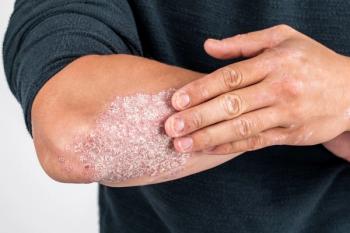
In September 2022, the FDA approved a new plaque psoriasis medication, deuvacracitinib (Sotyktu), and it may serve as a promising therapy for individuals with moderate-to-severe plaque psoriasis.
Plaque psoriasis is a
Psoriasis is best treated with a personalized approach to medicine by targeting the specific markers in an individual’s genes that puts them at risk for disease activity, according to an
Some of the markers that have been identified include Th1 (IL-2, IL-12, and IFN-g), macrophagic M1 (IL-1, IL-6, and TNF-a), and Th17 (IL-6 and IL-17) cytokines.1
When it comes to targeted treatment, most of the recent drugs have targeted IL-12/IL-23, IL-17, and TNF-a cytokines.1
Here are some of the biologics used for the treatment of moderate-to-severe plaque psoriasis:
- Ixekizumab (Taltz) and Secukinumab (Cosentyx), which target the IL-17A cytokine1
- Adalimubab (Humira), etanercept (Enbrel), and infliximab (Remicade), which target the TNF-a cytokines
- Stelara (Ustekinumab), which targets the IL-12 and IL-23 cytokines
Sotyktu is first-of-its-class
In a large, phase 3,
At week 16, participants receiving Sotyktu were found to have
Sotyku is
Counseling Points
Sotyktu is taken orally
Heterosexually active women who could become pregnant should be on an effective birth control method while taking this medication, to avoid the chance of pregnancy.3 Because this drug
The most common adverse effects of this medication are acne, folliculitis, mouth ulcers, nasopharyngitis, and respiratory infections.3,5 Pharmacists should not give live vaccines, such as herpes zoster, to patients taking Sotyktu. Because this medication suppresses the immune system, it is possible for patient to contract serious infections. All patients should be screened for tuberculosis, both active and latent, before starting therapy.3
Financial Barriers
Sotyktu comes with a large price tag, around $6501 for a supply of 30 tablets, according to
This will most likely be cost prohibitive for the general patient population.6 There may be discount programs available through the manufacturer.6 Until the cost goes down, and this drug becomes readily available in all pharmacies, the best treatment options for patients continues to be those that are accessible, covered by insurance, and safe.
Clinical Trial Limitations
As a caveat, it must be noted that this large study had several conflicts of interest, including investigators who were receiving grant and financial support from the pharmaceutical companies to conduct this research, as well as serving as consultants for these companies, which may ultimately have favored the results in favor of this new drug, despite the double blinding.
References
1. Camela E, Potestio L, Fabbrocini G, Ruggiero A, Megna M. New frontiers in personalized medicine in psoriasis. Expert Opin Biol Ther. 2022;22(12):1431-1433. doi:10.1080/14712598.2022.2113872
2. Kanda N. Psoriasis: pathogenesis, comorbidities, and therapy updated. Int J Mol Sci. 2021;22(6):2979. doi:
3. Sotytku. Prescribing information. Bristol-Myers Squibb Company. 2022. Accessed May 8, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
4. Sotyktu. Bristol Myers Squibb. Accessed May 8, 2023.
5. Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51. doi:10.1016/j.jaad.2022.08.061
6. Sotyktu prices, coupons, and patient assistance programs. Drugs.com. Accessed April 10, 2023.
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.